Controlled release tablets based on HPMC:lactose blends
Hydroxypropyl methylcellulose (HPMC) is a frequently used matrix former for controlled release tablets. To adjust desired drug release kinetics, freely water-soluble lactose can be added.
The aim of this study was to investigate the importance of the type of preparation technique of propranolol HCl loaded matrix tablets based on such HPMC:lactose blends. The tablets were prepared by: (i) direct compression of physical blends of drug, HPMC and lactose, (ii) direct compression of blends of the drug and co-processed HPMC:lactose (spray agglomerated), and (iii) via wet granulation.
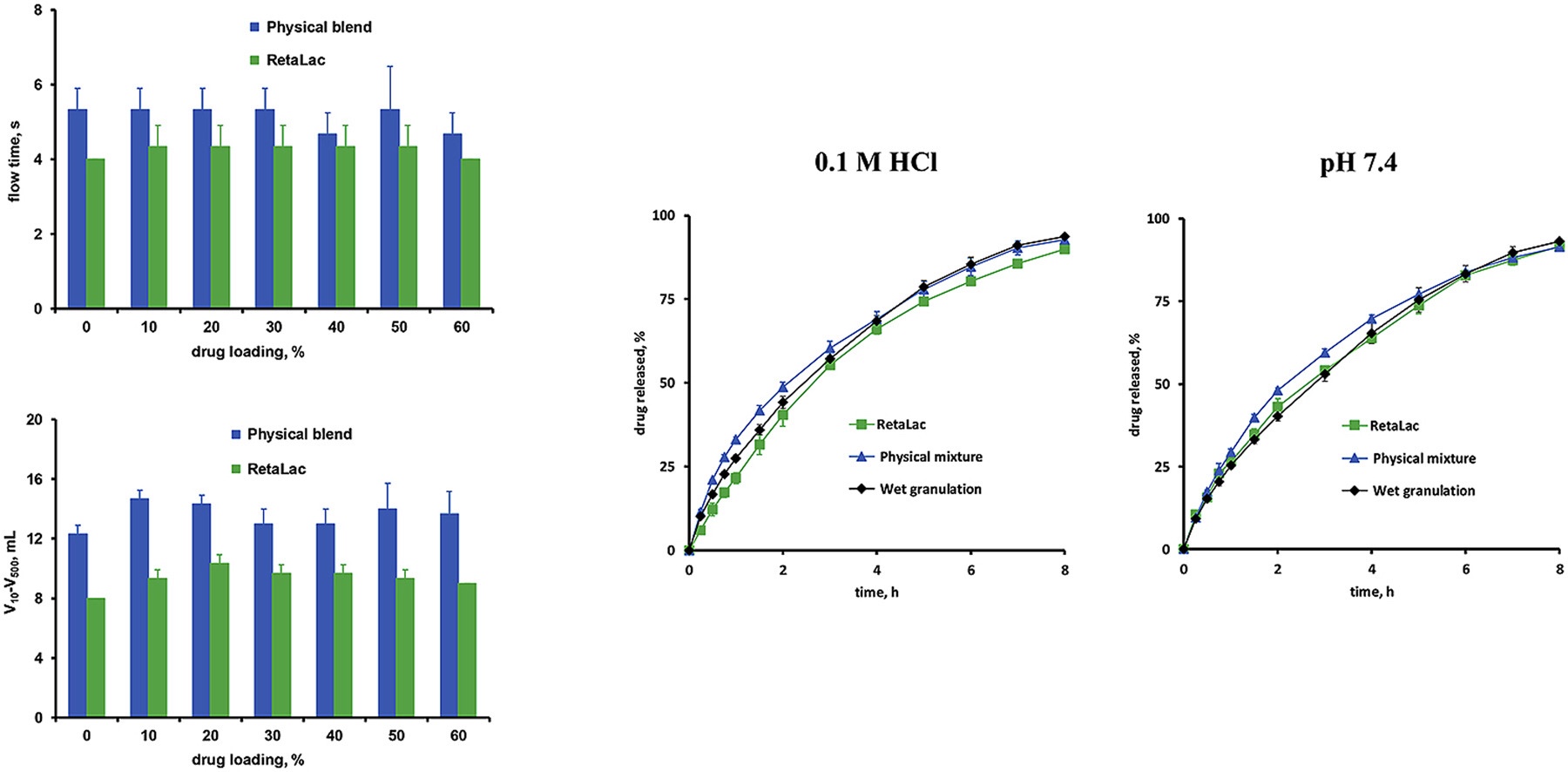
Interestingly, the co-processed HPMC: lactose particles provided better flowability and compactability than the physical HPMC:lactose blends, while the resulting water uptake kinetics and drug release rates were similar. Also wet granulation led to similar drug release kinetics. Thus, the use of co-processed HPMC:lactose can offer interesting technical advantages for the manufacturing of controlled release matrix tablets, while system performance remains unaltered. More on HPMC and lactose blends for controlled release tablets
Materials
Propranolol HCl (Fagron, Saint-Denis, France); α-lactose monohydrate (spray agglomerate, Tablettose 80) and “a 50:50 co-processed spray agglomerate of hydroxypropyl methylcellulose & α-lactose monohydrate” (RetaLac) (Meggle, Wasserburg, Germany); hydroxypropyl methylcellulose (HPMC, Methocel K4M Premium DC or Benecel K4M; Stobec, St-Jerome, Canada); magnesium stearate (Cooperation pharmaceutique francaise, Melun, France).
Conclusion
HPMC:lactose blends offer an interesting potential as matrix formers in controlled drug delivery systems. The type of preparation technique can strongly affect the key properties of the powder/granule blends, which are to be compressed (in particular their flowability and compactability). In contrast, the resulting water uptake and drug release kinetics are similar. This indicates that differences in the distribution of the drug, HPMC and lactose, resulting from the different manufacturing procedures, do not affect the conditions for drug release to a substantial extent. From a practical point of view, the use of spray agglomerated “HPMC:lactose” offers the advantage of improved flowability compared to physical HPMC:lactose mixtures.

