Experimental and Modeling Study of Drug Release from HPMC-Based Erodible Oral Thin Films
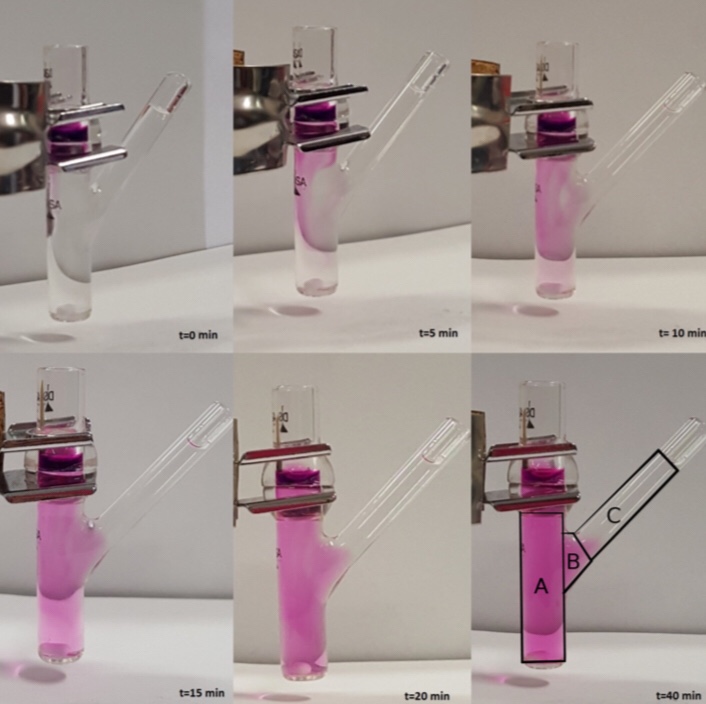
In this work hydroxypropyl methylcellulose (HPMC) fast-dissolving thin films for oral administration are investigated. Furosemide (Class IV of the Biopharmaceutical Classification System) has been used as a model drug for in vitro release tests using three different set-ups: the Franz cell, the millifluidic flow-through device, and the paddle type dissolution apparatus (USP II). In order to enable drug incorporation within HPMC films, a multifunctional excipient, hydroxypropyl-β-cyclodextrin (HP-β-CD) has been included in the formulation, and the influence of HP-β-CD on film swelling, erosion, and release properties has been investigated. Mathematical models capable of describing the swelling and release processes from HPMC erodible thin films in different apparatuses have been developed. In particular, we propose a new model for the description of drug transport and release in a Franz cell that accounts for the effect of the unavoidable imperfect mixing of the receptor chamber.
Download article here: pharmaceutics-10-00222.pdf

