Development of solid lipid nanoparticle as carrier of pioglitazone for amplification of oral efficacy
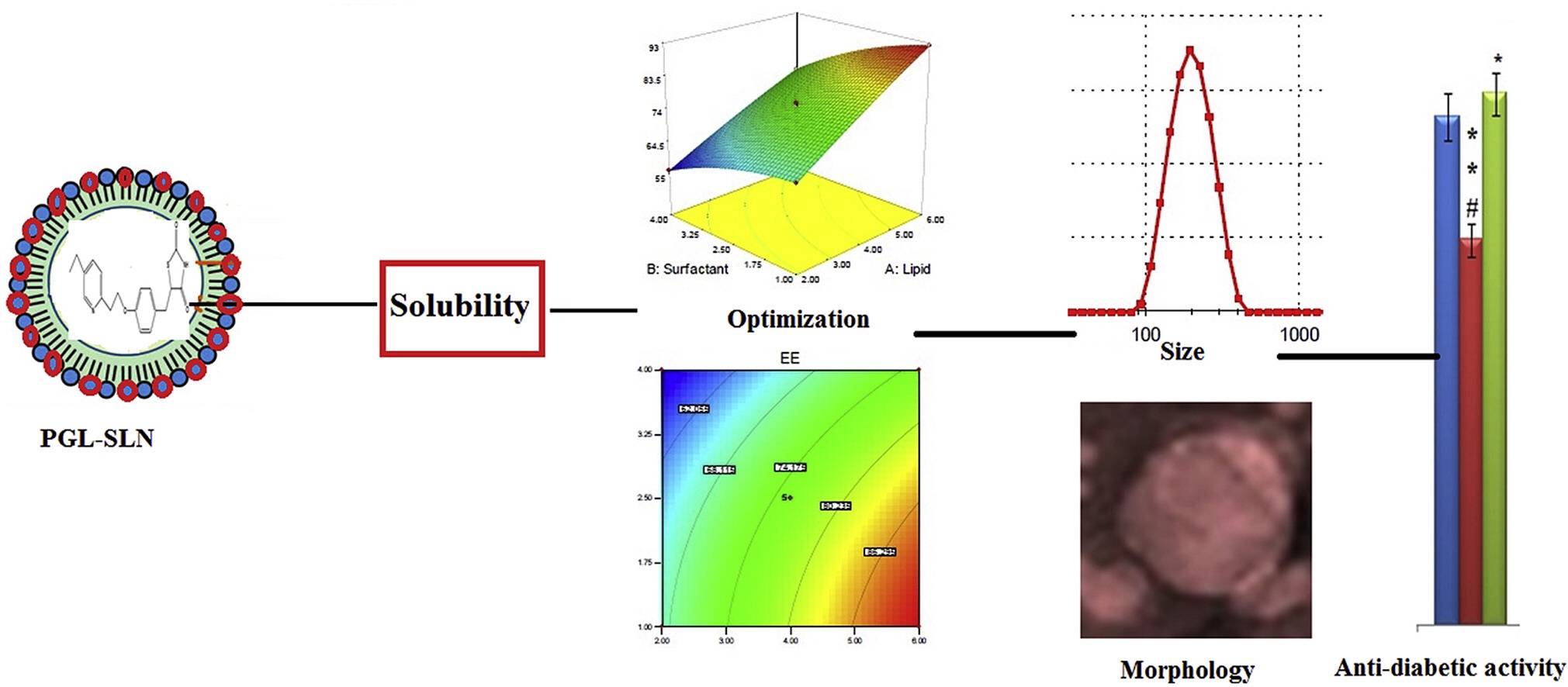
Pioglitazone (PGL) is a hypoglycemic therapeutic agent used in the treatment of diabetes (type 2). The present research work was designed to prepare and optimize PGL loaded solid lipid nanoparticles (SLNs). The formulation was optimized using three factors lipid (Compritol® 888 ATO – A), surfactant (tween80- B) and homogenization speed (C), and their effects were evaluated on particle size (Y1in nm) and encapsulation efficiency (Y2in %).
The optimized formulation (PGL-SLNopt) was further evaluated for physicochemical characterization, drug release, stability study, in–vivo antidiabetic and biochemical study. The optimized formulation PGL-SLNopt prepared with the composition of Compritol® 888 ATO (4.5% w/v), tween 80 (3.0% w/v) and homogenization speed (3800 rpm). The PGL-SLNopt hasshown particle size, EE, PDI, and zeta-potential 180.65 nm, 85.34%, 0.231 and −30.7 mV, respectively. The DSC and XRD spectra did not showed any peak of PGL confirm the complete entrapment of PGL in lipid due to solubilization. PGL-SLNopt exhibited significant (P < 0.05) prolonged release (89.56 ± 3.11% in 12 h) than pure PGL.
The anti-diabetic study indicated that PGL-SLNopt showed significantly (P < 0.05) higher hypoglycemic activity (57.6% reduction in 12 h) as well as improved biochemical parameters. Our finding results revealed that PGL-SLNs could be a potential delivery system for the management of diabetes (type-2). Continue on Development of solid lipid nanoparticle as carrier of pioglitazone

