Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery
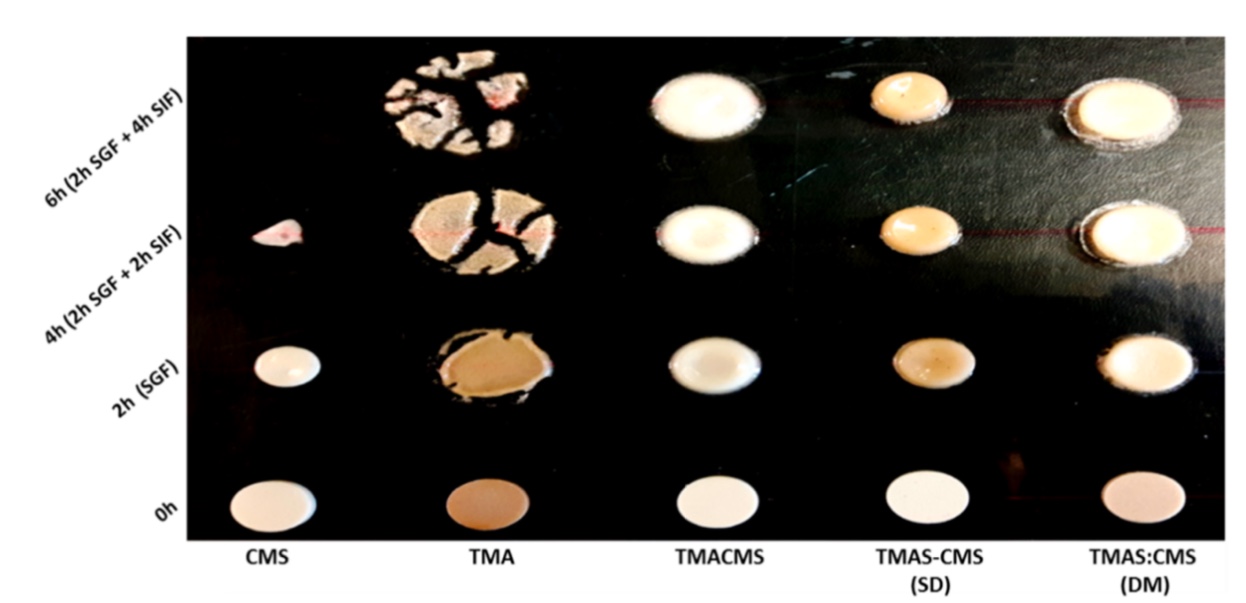
The potential of the polyampholytic and polyelectrolytic starch compounds as excipients for drug controlled release was investigated using various tracers differing in terms of solubility and permeability.
Ampholytic trimethylaminecarboxymethylstarch (TMACMS) simultaneously carrying trimethylaminehydroxypropyl (TMA) cationic groups and carboxymethyl (CM) anionic groups was obtained in one-step synthesis in aqueous media. Trimethylaminestarch (TMAS) and carboxymethylstarch (CMS) powders were also synthesized separately and then homogenized at equal proportions in liquid phase for co-processing by spray drying (SD) to obtain polyelectrolytic complexes TMAS-CMS (SD).
Similarly, equal amounts of TMAS and CMS powders were dry mixed (DM) to obtain TMAS:CMS (DM). Monolithic tablets were obtained by direct compression of excipient/API mixes with 60% or 80% drug loads. The in vitro dissolution tests showed that ampholytic (TMACMS) and co-processed TMAS-CMS (SD) with selected tracers (one from each class of Biopharmaceutical Classification System (BCS)), were able to control the release even at very high loading (80%). The presence of opposite charges located at adequate distances may impact the polymeric chain organisation, their self-assembling, and implicitly the control of drug release.
In conclusion, irrespective of preparation procedure, ampholytic and polyelectrolytic starch materials exhibited similar behaviours. Electrostatic interactions generated polymeric matrices conferring good mechanical features of tablets even at high drug loading. Download the full MDPI article here: ampholytic-and-polyelectrolytic-starch-as-matrices-for-controlled-drug-delivery.pdf

