All excipient production sites with GMP certification
In the pharmaceutical world, the term GMP, or Good Manufacturing Practice, plays quite a big role. In this article, we explain what GMP stands for in general, and specifically for the pharmaceutical excipient industry. Moreover, it is shown which organizations perform testing and inspection, and how the GMP-certification process works. To get a good overview of the currently GMP-certified excipient production sites, we have marked all of them in an interactive map with additional information available.
What is GMP exactly?
“Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. […] The World Health Organization (WHO) version of GMP is used by pharmaceutical regulators and the pharmaceutical industry in over 100 countries worldwide, primarily in the developing world. The European Union’s GMP (EU-GMP) enforces similar requirements to WHO GMP, as does the FDA’s version in the US. Similar GMPs are used in other countries, with Australia, Canada, Japan, Saudi Arabia, Singapore, Philippines, Vietnam and others having highly developed/sophisticated GMP requirements.” (Wikipedia)
In the excipient industry, EXCiPACT has an important role:
Regulators require excipient users to qualify their suppliers based on GMP/GDP audits and they have indicated that third party auditing of suppliers is acceptable if a creditable certification body issues certificates and audit reports by employing qualified auditors who are demonstrably credible in suitable GMP/GDP standards and in the needs of the pharmaceutical industry. EXCiPACT asbl is a non-profit organisation that owns and manages oversight of such an independent, high quality, third party Certification Scheme available to pharmaceutical excipient manufacturers and distributors worldwide.
Which certification bodies are working with EXCiPACT?
There are several certification bodies working with EXCiPACT, these are: SGS (global); AENOR (global); AJA (global), blue inspection body (global), DQS (mainly Europe and Mid-East); BV (mainly China), and Certiquality (mainly Italy).
The Certification Scheme can only be undertaken by a Registered EXCiPACT Certification Body employing a Registered EXCiPACT Auditor. The Certification process contains eigth steps in total.
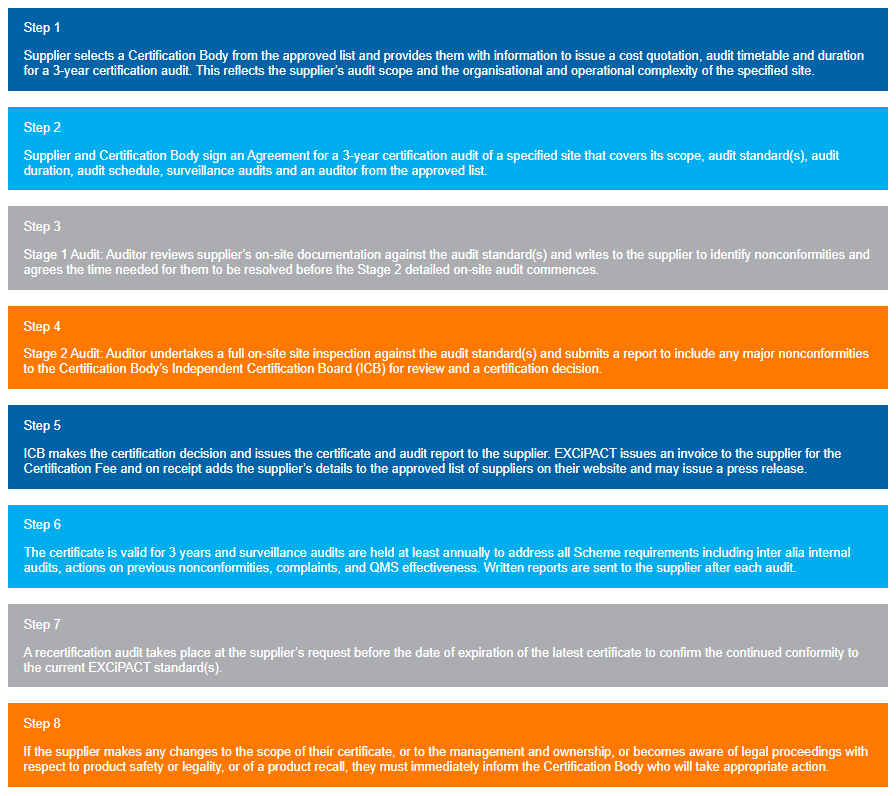
This is the first step:
“The Supplier selects a Certification Body from the approved list and provides them with information to issue a cost quotation, audit timetable and duration for a 3-year certification audit. This reflects the supplier’s audit scope and the organisational and operational complexity of the specified site.”
The remaining steps of the certification process can be seen here or in the graphic on the right side, which can be expanded.
The detailed process may vary with each Registered EXCiPACT Certification Body.
Developed by excipient suppliers and users, the Scheme’s core objectives are (i) to demonstrate a safe, reliable, transparent pharmaceutical supply chain, and (ii) to reduce the audit burden, costs and resources for manufacturers, distributors and users of pharmaceutical excipients without compromising quality. The Scheme comprises the following components:
- GMP standard for excipients as an annex to ISO 9001:2015
- GDP standard for excipients as an annex to ISO 9001:2015
- Certifying Body competency qualification as an annex to ISO 17021-1:2015
- Auditor competency qualification as an annex to ISO 17021-1:2015.
Have a look at all the pharmaceutical excipient production sites with EXCiPACT Certification here:
See the GMP-certified production site’s of our partners:
| Name | Certification Body | Site Location | Scope | Issue Date | Expiry Date | Registration Number | Certified Since |
|---|---|---|---|---|---|---|---|
| Air Liquide Austria GmbH | AJA (Global) | Schwechat, Austria | GDP, GMP | 19. Nov 19 | 19. Nov 22 | AJAEU/20/009 | 19. Nov 19 |
| Air Liquide Benelux Industries | DQS GmbH | Marchienne-au-Pont, Belgium | GDP, GMP | 02. Aug 19 | 01. Aug 22 | 527184 EXCI | 02. Aug 16 |
| Air Liquide France Industrie Grande Synthe | SGS | Grande-Synthe, France | GMP | 12 January, 2021 | 11 January, 2024 | FR17/81842681 | 12 January, 2018 |
| Air Liquide France Industrie Moissy Cramayel | SGS | Moissy Cramayel, France | GMP | 12 January, 2021 | 11 January, 2024 | FR17/81842665 | 12 January, 2018 |
| Air Liquide France Industrie, Feyzin Activité Liquide | SGS | Feyzin, France | GMP | 12 January, 2021 | 11 January, 2024 | FR17/81842646 | 12 January, 2018 |
| Air Liquide Italia Produzione srl | Certiquality | Castelnuovo del Garda, Verona, Italy | GMP | 11 December, 2020 | 10 December, 2023 | P4513 | 11 December, 2020 |
| Air Liquide Italia Produzione srl | Certiquality | Limito Di Piotello, Milan, Italy | GMP | 19. Nov 20 | 18. Nov 20 | P4485 | 19. Nov 20 |
| Air Liquide Italia Service SRL | Certiquality | Rodano (Milano), Italy | GMP | 25. Aug 20 | 24. Aug 20 | P4452 | 25. Aug 20 |
| AL Air Liquide España, S.A. | AENOR | Cabanillas del Campo (Guadalajara), Spain | GDP, GMP | 16. Sep 20 | 16. Sep 23 | EXC-2020/001 | 16. Sep 20 |
| AL Air Liquide España, S.A. | AENOR | Madrid, Spain | GDP, GMP | 16. Sep 20 | 16.09.2020 | EXC 2020/002 | 16. Sep 20 |
| Armor Proteines S.A.S. (ARMOR PHARMA) | SGS | Loudéac, France | GMP | 08. Aug 19 | 07. Aug 22 | FR16/1842321 | 08. Aug 19 |
| BASF Advanced Chemicals Co., Ltd | Bureau Veritas China | Pudong, Shanghai, PR China | GMP | 05 March, 2020 | 04 March, 2023 | CNBJ202001 | 05 March, 2020 |
| BIOGRUND GmbH | DQS GmbH | Hünstetten, Germany | GDP, GMP | 26 June, 2019 | 25 June, 2022 | 296948 EXCI | 13 December, 2013 |
| Chemische Fabrik Budenheim KG | SGS | Budenheim, Germany | GDP, GMP | 14. Apr 20 | 13. Apr 23 | DE16/819942543 | 19 June, 2014 |
| Evonik Nutrition & Care GmbH | DQS GmbH | Darmstadt, Weiterstadt, Germany | GDP, GMP | 08 January, 2020 | 07 January, 2023 | 527392 EXCI | 27 January, 2014 |
| Ideal Cures Private Limited | SGS | Jammu, Jammu & Kashmir, India | GMP | 12 February, 2021 | 04 February, 2024 | IN/EXCiPACT/15/50053 | 06 February, 2015 |
| Ideal Cures Private Limited | SGS | Vasai (East), Palghar, India | GMP | 27. Apr 18 | 26. Apr 21 | IN/EXCiPACT/15/50060 | 27. Apr 15 |
| JRS Pharma | SGS | Cedar Rapids, Iowa, USA | GMP | 27. Aug 19 | 26. Aug 22 | US19/819943331 | 27. Aug 19 |
| JRS Pharma & Gujarat Microwax Private Limited | SGS | Nandasan, India | GMP | 19. Aug 18 | 18. Aug 21 | IN18/500165 | 19. Aug 18 |
| JRS Pharma & Gujarat Microwax Private Limited | SGS | Nandasan, India | GMP | 19. Aug 18 | 18. Aug 21 | IN18/500165.01 | 19. Aug 19 |
| JRS Pharma and Gujarat Microwax Pvt. Ltd. (Unit - IV) | SGS | Mehsana, Gujarat, India | GMP | 17 December, 2020 | 17 December, 2023 | IN20/818844536 | 17 December, 2020 |
| JRS Pharma Oy | AJA (Global) | Nastola, Finland | GMP | 27 January, 2020 | 27 January, 2023 | AJAEU/15850 | 27 January, 2020 |
| MEGGLE GmbH & Co. Kg | SGS | Wasserburg, Germany | GDP, GMP | 01 July, 2020 | 30 June, 2023 | DE17/819943082 | 27 July, 2014 |
| Merck KGaA | blue inspection body | Darmstadt, Germany | GDP, GMP | 17 June, 2019 | 17 June, 2022 | E-ME-18887-2019-01 | 25 June, 2013 |
| Merck Life Science Technologies (Nantong) Co., Ltd. | Bureau Veritas China | Nantong, Jiangsu, PR China | GMP | 30 June, 2020 | 29 June, 2023 | CNSH7682741 | 30 June, 2020 |
| Microcellulose Weissenborn GmbH & Co KG | DQS GmbH | Weissenborn/Erzgebirge, Germany | GDP, GMP | 15 January, 2019 | 14 January, 2022 | 537607 EXCI | 15 January, 2019 |
| Nutrition & Biosciences USA 1 LLC Midland Ethocel Operations | SGS | Midland, Michigan, USA | GMP | 07 January, 2018 | 06 January, 2021 | US18/81827258 | 07 January, 2018 |
| Nutrition & Biosciences USA 1 LLC Midland Methocel Operations | SGS | Midland, Michigan, USA | GMP | 08 January, 2018 | 07 January, 2021 | US18/81827257 | 08 January, 2018 |
| Nutrition & Biosciences USA 1, LLC LA Methocel Operations | SGS | Plaquemine, Louisiana, United States | GMP | 26 June, 2018 | 25 June, 2021 | US18/81827450 | 26 June, 2018 |
| Omya SAS | Bureau Veritas China | Orgon, France | GMP | 23 January, 2019 | 22 January, 2022 | CNSH10344096 | 01 January, 1970 |
| Rettenmaier Iberica SL y Cia S.C | AENOR | Barcelona, Spain | GDP | 12 January, 2021 | 12 January, 2024 | EXC 2021/001 | 12 January, 2021 |
| Rettenmaier Natural Fiber Manufacturing (Changzhou) Co., Ltd. | Bureau Veritas China | Changzou, PR China | GMP | 17 July, 2020 | 16 July, 2023 | CNSH6866459 | 17 July, 2020 |
| ROQUETTE FRERES | SGS | Lestrem, France | GMP | 24 December, 2018 | 23 December, 2021 | FR15/81841993 | 24 December, 2015 |
| SEPPIC | SGS | Castres, France | GMP | 04. Sep 18 | 03. Sep 21 | FR16/81842387 | 22. Nov 13 |
| SEPPIC (Shanghai) Chemical Specialities Co., Ltd | SGS | Shanghai, China | GMP | 24 July, 2019 | 23 July, 2022 | CN16/21698 | 22. Nov 16 |
| Specialty Products US, LLC | SGS | Institute, West Virginia, USA | GMP | 07 January, 2018 | 06 January, 2021 | US18/81827245 | 07 January, 2018 |
Sources: Wikipedia, EXCiPACT, EXCiPACT 2


