Nagase Viita – Pharmaceutical Ingredients
― A world leading supplier of pharmaceutical grade Trehalose ―
Founded as a starch syrup manufacturer in 1883, Nagase Viita (Formerly Hayashibara Co., Ltd.) has grown into a company with strong research and development expertise, engaging in original and creative research. Utilizing our technological background in biotechnology and in-depth knowledge of saccharide production, Nagase Viita became the first company in the world to succeed in the mass production of “Trehalose”, a unique disaccharide recognized as a beneficial excipient for the biopharmaceutical industry. In addition to trehalose, Nagase Viita has been supplying for many years other high purity ingredients to the pharmaceutical industry worldwide.
― Nagase Viita’s constant goal is to be a valued supplier in biopharmaceutical development ―
OUR STRENGTHS:
- High purity, Low endotoxin grade
- Experience in industrial saccharide production
- Integrated production of high purity products starting from saccharide production to purification (endotoxin removal) in our factory in Japan
- Compliance with all major pharmacopoeias
- EcoVadis rating in sustainability since 2020

Nagase Viita has been committed to sustainable manufacturing, considering the well-being of people and natural environment as our core importance. We have been awarded the platinum rating, the top 1% of 130,000 assessed companies by EcoVadis, the world’s most trusted assessment organization in France for business sustainability ratings.
For Parenteral Biopharmaceuticals
Our Unique Brand for Biopharmaceuticals
SOLBIOTETM
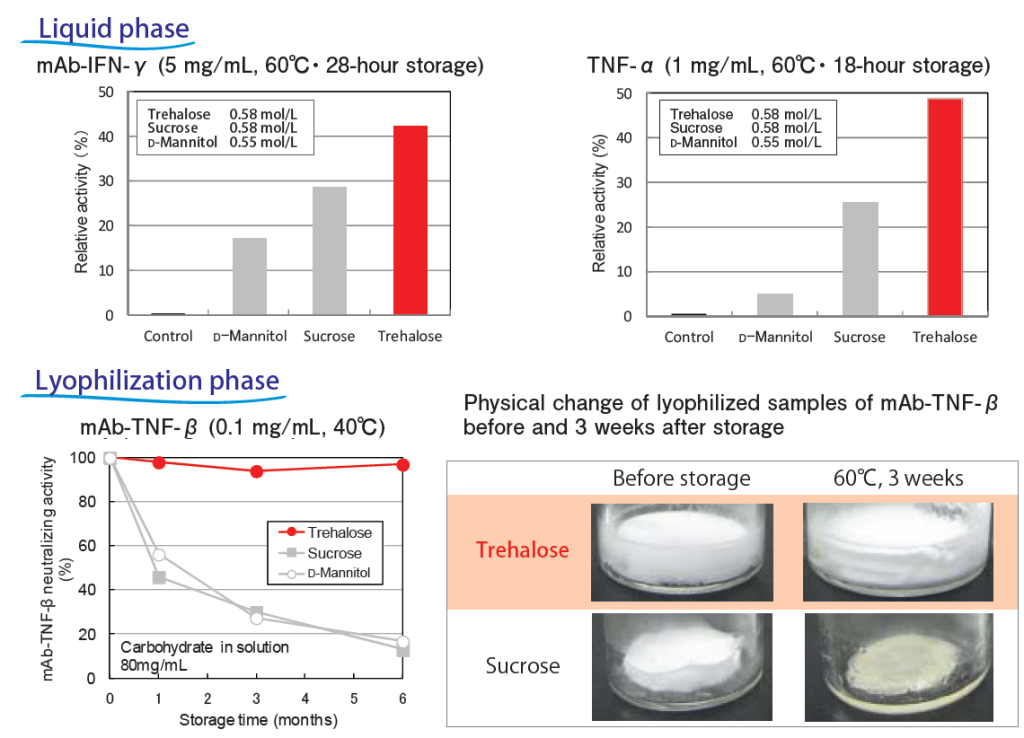
SOLBIOTETM was launched with the specific aim of delivering a sustainable solution to biopharmaceutical development. It features two high-purity and low-endotoxin saccharide excipients, Trehalose SG and Maltose PH.
SOLBIOTETM is making an important contribution to biopharmaceutical drug development, which is enabling manufactures to maintain the high quality of drugs during storage by significantly improving the stability of protein APIs such as antibodies, fusion proteins and immunoglobulins.

― High Purity Trehalose ―
Trehalose SG
Trehalose is a dihydrate crystalline non-reducing disaccharide consisting of two glucose molecules linked by an α,α‒1,1 bond. Trehalose SG has been supplied and used for parenteral formulations because of its high purity and low endotoxin specification. Trehalose is unique in its remarkable acid tolerance as well as heat stability and shows no Maillard reaction when heated in solutions containing amino acids. It shows several benefits in stabilizing protein structures when proteins are lyophilized or frozen, and is also known to prevent cells and liposomes from damage during freezing and thawing.
APPLICATIONS
- Stabilization of antibodies, peptides, and vaccines for injection
- Stabilization of exosomes
- Additive for culture medium (e.g., stem cells)
- Preservation of platelets
REGULATORY APPROVALS
- JP / USP-NF / Ph.Eur. / CP
- US Type IV DMF, Chinese DMF
- Kosher, Halal
CHARACTERISTICS
― High Purity Maltose ―
Maltose PH
Maltose is a reducing disaccharide consisting of two glucose molecules linked by an α-1,4 bond. Maltose PH is highly purified maltose monohydrate crystalline with a low endotoxin specification. It can be used for parenteral applications including injection, intravenous fluid, and cell culture carbon sources.
APPLICATIONS
- Active ingredient for infusion fluid
- Solubility enhancer and stabilizer of freeze-dried anti-cancer agents for injection
- Stabilizer of Immunoglobulins
REGULATORY APPROVALS
- JP/ CP
- US Type II DMF
- Kosher, Halal
CHARACTERISTICS
For Oral Drug Development
Sugar excipients are often used in solid oral dosage forms such as tablets. They are used for different purposes including taste improvement, tableting and granulation.

― High Purity Trehalose ―
Trehalose 100PH
Trehalose 100PH is a highly purified trehalose without an endotoxin specification. Since it shows great benefit in masking unpleasant taste and odor, Trehalose 100PH can be used in syrup, dry syrup as well as chewable tablets, making it a more preferred additive for oral drug development.
APPLICATIONS
- Additive for oral solutions
(e.g., stabilizer, taste improver, solubility enhancer for minerals) - Excipient for solid oral dosage forms
(e.g., tablets)
REGULATORY APPROVALS
- JP / USP-NF / Ph.Eur.
- Kosher, Halal
Brochures:
Contact
NAGASE & CO., LTD.
Life & Healthcare Products Department
Pharmaceutical Ingredients | Contact us | Nagase Viita Co., Ltd.












