Comparative study on disintegration methods for oral film preparations
13. September 2018
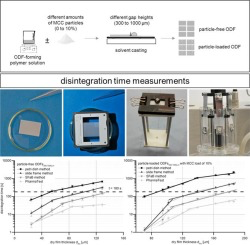
Orodispersible films (ODFs) provide high application comfort due to rapid disintegration in the oral cavity. They increasingly found the approval of pharmaceutical research and development and were added to the European Pharmacopeia in 2012. The European Pharmacopeia explicitly demands disintegration testing for ODFs, but does not refer to a suitable method. The aim of this study was to collect and evaluate existing disintegration methods regarding their suitability to investigate different ODF formulations. Therefore, 21 ODF formulations produced at different gap heights and/or different amounts of suspended microcrystalline cellulose (MCC) particles were manufactured by solvent casting technique, using hypromellose (HPMC) as film-forming polymer. Four disintegration methods described in literature were applied to characterize the disintegration behavior of these formulations. They were the petri dish, the slide frame, slide frame and ball (SFaB) method as well as the PharmaTest® disintegration tester equipped with a film sample holder. All methods show similar tendencies, at which the disintegration time proportionally increases with increasing dry film thicknesses. Reduced disintegration times were observed for ODFs containing insoluble MCC particles compared to their corresponding particle-free formulations. However, the suitability to investigate varying types of ODFs applying the four different test methods highly depends on the intended purpose. Therefore, the slide frame and SFaB method seems to be particularly applicable for research and development purposes, whereas the PharmaTest® disintegration tester and the SFaB method fulfill the demands required for testing methods within the quality control.