Impact of Critical Material Attributes (CMAs)-Particle Shape on Miniature Pharmaceutical Unit Operations
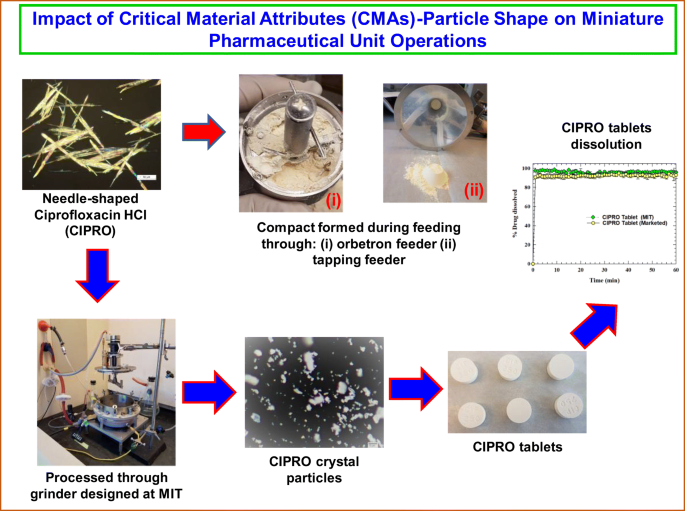
The U.S. Food and Drug Administration (FDA) emphasizes drug product development by Quality by Design (QbD). Critical material attributes (CMAs) are a QbD element that has an impact on pharmaceutical operations and product quality. Pharmaceutical drugs often crystallize as needle-shaped (a CMA) particles and affect the process due to poor flowability, low bulk density, and high compressibility, and eventually the product performance.
In this study, the product obtained from crystallization was needle-shaped Ciprofloxacin HCl (CIPRO), formed lumps during drying, and compacted during processing through feeders. To delump small amounts of materials and break the needles, multiple available devices (mortar-pestle, Krups grinder) and custom-made grinder were assessed before formulation. The processed CIPRO powder was then used to make tablets in the miniature tablet manufacturing unit developed by the team at MIT. The critical quality attributes (CQA) of the tablets, set by the United States Pharmacopeia (USP), were then assessed for the drug powder processed with each of these devices.
Powder properties comparable to commercial CIPRO were obtained when the custom MIT-designed grinder was used, leading to tablets that meet the USP criteria, with comparable dissolution profiles of those for marketed CIPRO tablets. This study demonstrates how needle-shaped crystals have an impact on pharmaceutical operations, even if it is on a miniature scale, and how proper shape and subsequent flow properties can be obtained by processing the particles through the MIT team-designed grinder.
Download the full article here: Impact of Critical Material Attributes (CMAs)-Particle Shape on Miniature Pharmaceutical Unit Operations
or continue reading here: Azad, M.A., Capellades, G., Wang, A.B. et al. Impact of Critical Material Attributes (CMAs)-Particle Shape on Miniature Pharmaceutical Unit Operations. AAPS PharmSciTech 22, 98 (2021). https://doi.org/10.1208/s12249-020-01915-6
Materials
Ciprofloxacin hydrochloride monohydrate (CIPRO, 99.8% purity) was purchased from Jai Radhe Sales (Gujrat, India) and used as the model drug. Bulk density, compression, biopharmaceutical properties, stability/target shelf life, etc., determines the selection of excipients to design the drug product. Stability improvement excipients were not considered as manufacturing is an on-demand basis. A simplified method in formulation development was deliberated by reducing the number of excipients necessary for tableting. Hence, to formulate only one filler/diluent, flow aid/glidant, and lubricant were chosen. Silicified microcrystalline cellulose (Prosolv SMCC® HD 90) was gifted by JRS Pharma GMBH & CO. KG (Rosenberg, Germany) and considered as the filler/diluent, fumed silica (CAB-O-SIL® M-5P) was gifted by Cabot Corporation (MA, USA) and considered as the Glidant, and Magnesium stearate (Kosher Passover HyQual™), gifted by Mallinckrodt Pharmaceuticals (MO, USA), was considered as the lubricant. Sodium starch glycolate (EXPLOTAB®) gifted by JRS Pharma GMBH & CO. KG (Rosenberg, Germany) was used as a disintegrant to improve the disintegration of CIPRO tablets, as this process yields a volume dosage form that requires a higher compaction force than the reported earlier work with the drugs used in the unit (17). SSG also improves subsequent dissolution
Conclusions
Particle shape can impact powder flow behavior. Pharmaceutical unit operations are sensitive to particle shape, especially for systems presenting high aspect ratios. Grinding is very well-known in the pharmaceutical industry. However, to handle a small amount of powder, there is no miniature equipment available commercially. At MIT, we designed a grinder integrated with a filter dryer that produces dry CIPRO powder with optimum flow properties which are suitable for tableting. The tablet properties meet USP criteria and the dissolution profile is comparable to commercial CIPRO tablets. The overall findings, comparing this system to common alternatives, demonstrate that needle shape has an impact on pharmaceutical processing even on a miniature scale. These challenges have been overcome by the MIT-designed grinder.

