Quality by Design Applied Development of Immediate-Release Rabeprazole Sodium Dry-Coated Tablet
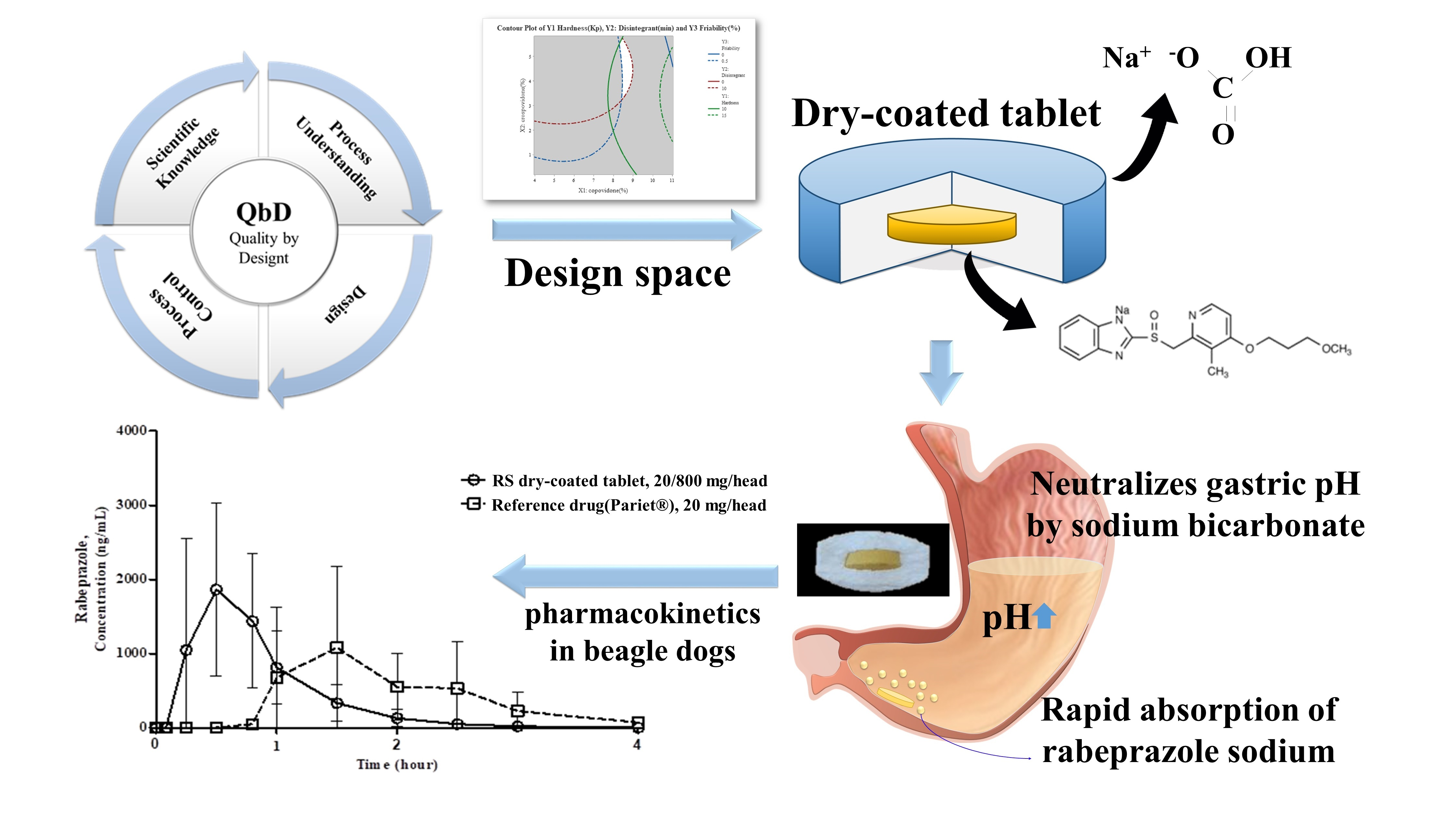
The aim of this study was to develop immediate-release oral rabeprazole sodium tablets with rapid efficacy and gastric stability for the treatment of gastroesophageal reflux disease. Rabeprazole sodium is a commonly prescribed proton pump inhibitor; however, it is extremely unstable and degrades in acidic environments. Hence, it has been manufactured and supplied only in enteric-coated tablet form, while immediate-release (IR) formulations for this drug are very limited. In this study, we applied the quality by design (QbD) approach to formulate and optimize an IR dry-coated tablet containing rabeprazole sodium as an inner core with an outer sodium bicarbonate layer to stabilize the active pharmaceutical ingredient at gastric pH.
We also investigated the stability of the pharmaceutical dosage form and its pharmacokinetic profile. The results show that the developed tablets are stable for approximately 12 months and have a high dissolution rate, greater than or equal to 90% at 30 min. Further, in vivo beagle pharmacokinetics confirmed that the newly developed IR tablet had an AUCt which is bioequivalent to the existing delayed-release rabeprazole tablet; however, its Tmax was 0.5 h, which is up to seven times faster than that of the existing tablet. Moreover, the IR tablet was found to immediately absorb in the stomach. Hence, the development of IR tablets can be used as a platform to overcome the technical and commercial limitations currently associated with various proton pump inhibitors used to treat patients with gastroesophageal reflux disease that require immediate therapeutic relief.
Download the full article here: Quality by Design Applied Development of Immediate-Release Rabeprazole Sodium Dry-Coated Tablet
or continue reading here: Lee, S.-H.; Kim, J.-E. Quality by Design Applied Development of Immediate-Release Rabeprazole Sodium Dry-Coated Tablet. Pharmaceutics 2021, 13, 259. https://doi.org/10.3390/pharmaceutics13020259
Materials
The active pharmaceutical ingredient (API) rabeprazole sodium was purchased from Ildong (Seoul, Korea), while sodium bicarbonate was purchased from Hebei Huachen Pharmaceutical Co. Ltd. (Hebei, China). Additionally, the following reagents were used throughout the study: D-mannitol (Roquette, Lestrem, France), heavy calcium carbonate (Shanghai Nuocheng Pharmaceutical Co. Ltd., Shanghai, China), magnesium oxide (Tomita Pharmaceutical Co. Ltd., Tokushima, Japan), calcium hydroxide (Spectrum Pharmaceuticals, Henderson, NV, USA), hydroxypropyl cellulose (Nippon Soda Co. Ltd., Tokyo, Japan), sodium starch glycolate (JRS Pharma, Rosenberg, Germany), lowsubstituted hydroxypropyl cellulose and ethylcellulose (Ashland, Covington, KY, USA), magnesium stearate (Faci Asia Pacific Pty Ltd., Jurong Island, Singapore), talc (Nippon Talc Co. Ltd., Osaka, Japan), titanium dioxide (Huntsman Corporation, Oulsburg, Germany), aluminum lake yellow No. 4 (Borak, Hwaseong, Gyeonggi, Korea), as well as copovidone and crospovidone (BASF Co. Ltd., Ludwigshafen, Germany). All other chemicals were of analytical reagent grade and purchased commercially.

