COVID-19 vaccine development and a potential nanomaterial path forward

The COVID-19 pandemic has infected millions of people with no clear signs of abatement owing to the high prevalence, long incubation period and lack of established treatments or vaccines. Vaccines are the most promising solution to mitigate new viral strains. The genome sequence and protein structure of the 2019-novel coronavirus (nCoV or SARS-CoV-2) were made available in record time, allowing the development of inactivated or attenuated viral vaccines along with subunit vaccines for prophylaxis and treatment. Nanotechnology benefits modern vaccine design since nanomaterials are ideal for antigen delivery, as adjuvants, and as mimics of viral structures. In fact, the first vaccine candidate launched into clinical trials is an mRNA vaccine delivered via lipid nanoparticles. To eradicate pandemics, present and future, a successful vaccine platform must enable rapid discovery, scalable manufacturing and global distribution. Here, we review current approaches to COVID-19 vaccine development and highlight the role of nanotechnology and advanced manufacturing. Read the full nature nanotechnology review article here or have a look at some “outtakes” of this article on vaccine design and strategies here:
Components and options in vaccine design
Antigen: a foreign material that can induce an immune response within the body—often derived from the pathogen one aims to immunize against. Based on how the antigen is presented, vaccines can be categorized as:
- Live-attenuated vaccine: weakened form of pathogens capable of replication, but not causing illness.
- Inactivated vaccine: killed form of pathogens incapable of replication or infection.
- Subunit vaccine: minimal antigenic element of a pathogen, for example, a protein, protein subunit or polysaccharides or VLPs self-assembled from these components. These antigens in purified forms are administered in combination with molecular adjuvants or expressed in vivo using RNA, DNA or viral vectors.
- Peptide-based vaccines: peptides are fundamental element of a protein subunit recognized by the immune system; all antigens described above contain peptide epitopes.
Adjuvant: a stimulatory agent designed to boost immune response toward a co-delivered antigen.
- Occurs as ‘independent entities’ in a mixture with antigens.
- Occurs as ‘conjugate-entities’ via chemical fusion directly to antigens.
Nanoparticle/nanocarrier: The live-attenuated and inactivated viral vaccines can be regarded as nanoparticles themselves. Rather than serving as the vaccine itself, a nanoparticle (viral or non-viral) can be employed as nanocarrier to encapsulate or present the antigen payload or nucleic acid encoding the antigen. Nanocarriers provide stability and targeting of these payloads to antigen presenting cells (APCs); nanocarriers can confer innate adjuvant behaviour (see Fig. 3). Nanocarriers synchronize delivery of both, antigen and adjuvant, to target immune cells.
- Viral vector: repurposed mammalian viruses engineered to deliver a gene encoding the antigen (examples include adenoviral vectors derived from chimpanzee and human).
- Proteinaceous nanoparticles: nanoscale biomaterial assemblies with atomic precision and complexity (examples include protein nanocages and non-infectious viruses such as plant viruses or bacteriophages) engineered to present a subunit vaccine or deliver a nucleic acid encoding the antigen.
- Synthetic nanoparticles: nanoscale assemblies of synthetic materials (examples include polymer, liposomal, or lipid nanoparticles) engineered to present a subunit vaccine or deliver a nucleic acid encoding the antigen.
Device: a piece of equipment designed to administer vaccine (Fig. 4).
- Syringe: hypodermic needle used for intramuscular, subcutaneous or intradermal delivery of vaccine by a healthcare professional (>10 mm length and 0.25–0.5 mm in outer diameter, somewhat invasive)
- Implant: slow-release device containing vaccine for sustained subcutaneous delivery, administered by a healthcare professional (<10 mm in length and <2 mm in width, more invasive)
- Microneedle patch: array of micrometre-scale needles containing vaccine for slow release, sustained intradermal delivery, administered by a healthcare professional or via self-administration (<1 mm in length and 0.1–0.5 mm in width, approximately 1 cm2 patch, minimally invasive).
The vaccine strategies
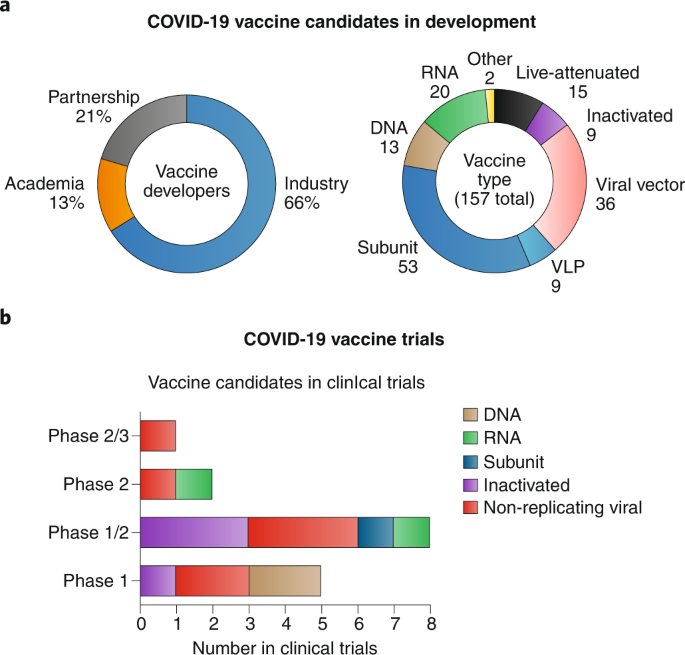
When designing a vaccine, principally, one needs to define the antigen, the adjuvant, the manufacturing system and the delivery strategy (Box 1). The rapid development of vaccines is possible because the genome and structural information of SARS-CoV-2 was made available in record time. These data, along with expedited communication of bioinformatic predictions and epitope mapping has provided crucial knowledge enabling vaccine design beyond development of live-attenuated and inactivated vaccines. Also, information available from prior development of SARS/MERS vaccine candidates aids in the development of SARS-CoV-2 vaccine candidates. Nanotechnology platforms offer great utility in modern vaccine design and have helped catalyse novel candidate vaccines toward clinical testing at unprecedented speed. Along with inactivated vaccines, emerging nanotechnologies such as mRNA vaccines delivered by lipid nanoparticles and viral vector vaccines have already reached Phase II and III clinical trials. Read the full nature nanotechnology review article here
Shin, M.D., Shukla, S., Chung, Y.H. et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. (2020). https://doi.org/10.1038/s41565-020-0737-y
The following article could also be of interest: