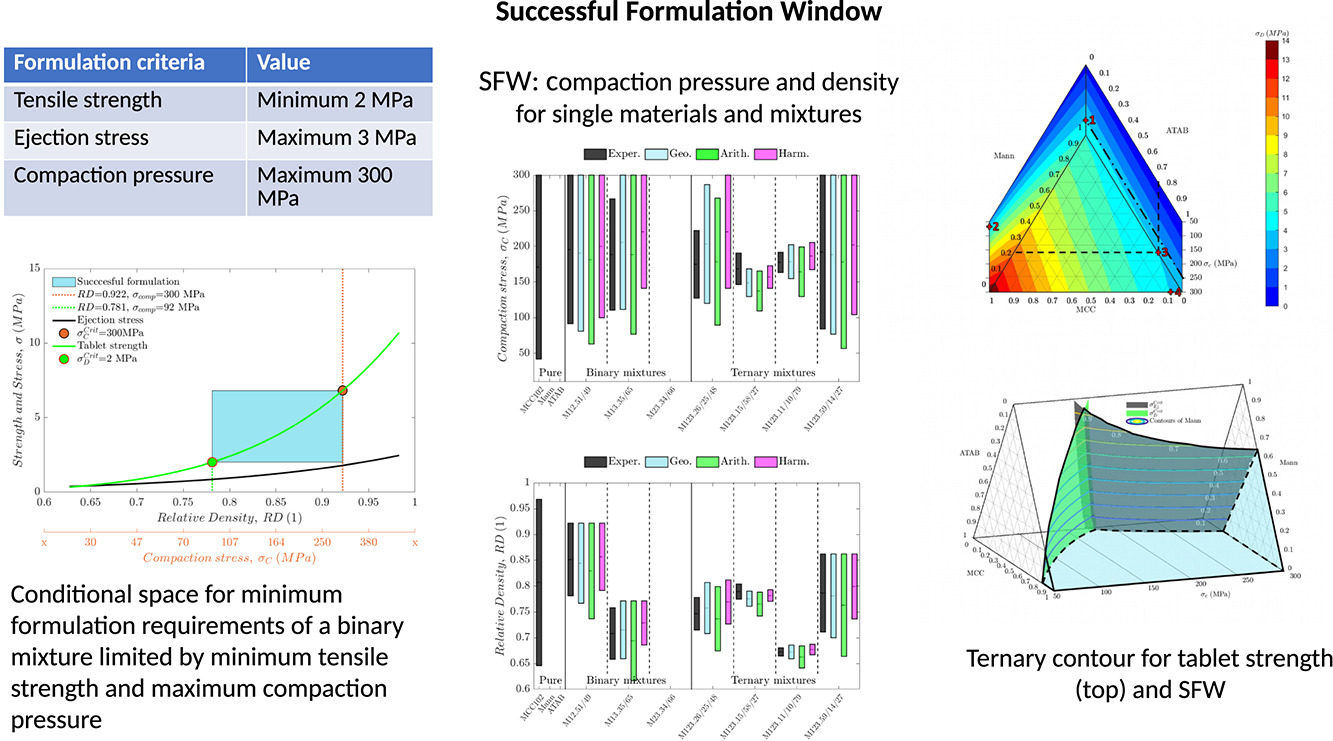
Successful Formulation Window for the design of pharmaceutical tablets with required mechanical properties
Abstract
Pharmaceutical tablet formulations combine the active ingredient with processing aids and functional components. This paper evaluates compressibility based predictive models for binary and ternary formulations to establish an acceptable range of tablet compression parameters that satisfy prescribed quality target criteria for tablets including minimum tablet strength and processing constraints such as maximum ejection stress and maximum compaction pressure. The concept of Successful Formulation Window (SFW) is introduced. A methodology is proposed to determine the SFW for a given formulation based on compaction simulator data collected for individual formulation components. The methodology is validated for binary and ternary mixtures and lubricated formulations. The SFW analysis was developed to support tablet formulation design to meet mechanical requirements.
1. Introduction
The popularity of tablets as a drug delivery system stems from advantages including low cost, long term storage stability, good tolerance to temperature, efficient manufacturing and ease of use by patients. Tablet manufacturing is a unit operation where a bulk powder material is compacted in a die using two opposing compression punches until the compact satisfies prescribed dissolution/disintegration and mechanical requirements. Powder formulations include the active pharmaceutical ingredient (API) and excipients which are pharmacologically inactive substances such as binders, diluents, disintegrants, lubricants and other processing aids necessary to achieve the desired bioavailability of the drug and physico-mechanical properties of tablets. Excipients can make up a significant proportion of the volume of tablet formulations. Excipients are derived from various sources such as animal (e.g. gelatine, lactose, stearic acid, etc.), plant (arginates, cellulose, starches, sugar, etc.), mineral (e.g. calcium phosphate, silica, etc.) and synthetic (e.g. polysorbates, povidone, etc.). Further information of origin, source and functionality can be found in Pifferi and Restani (2003) and Sheskey et al. (2020). Binders and diluents are used to add bulk, increase the strength of the tablets or improve flow properties (Arndt and Kleinebudde, 2018). Their rational selection in compositions influences the stability and bioavailability of the medicine (Reddy et al., 2013). Lubricants represent a particularly important class of pharmaceutical excipients which enable tablet manufacturing by reducing the friction between tooling and the powder material (Paul and Sun, 2018), thus reducing the compaction and ejection forces (Uzondu et al., 2018) or increase tablet brittleness (Paul and Sun, 2017a). However, the use of lubricants in formulations typically has a negative influence on tablet strength (Jarosz and Parrott, 1984, Miller and York, 1988) and tablet disintegration time (Shotton and Lewis, 1964). One of the most widely used lubricant in pharmaceutical tablet formulation is Magnesium Stearate (Miller and York, 1988). Lubricants may also prevent the adhesion of material to the punches (known as sticking) and minimize punch wear (Zuurman et al., 1999, Mitrevej and Augsburger, 1982). However, Magensium Stearate does not always prevent sticking (Roberts et al., 2004). Lubrication theory generally distinguishes two main types of lubrication (1) hydrodynamic or fluid lubrication, where the moving surfaces are separated by a layer of lubricant of a given viscosity, (2) boundary lubrication, where a thin film of lubricant separates the surfaces in contact influencing (a) friction, by supporting the interfacial load and (b) cohesion formed at particle–particle asperities or/and adhesion formed by particle-tooling asperities which penetrate the lubricant layer (Bowden and Tabor, 1967, Roblot-Treupel and Puisieux, 1986, Miller and York, 1988). Typical lubricants used in pharmaceutical formulations are boundary lubricants which are chemically inert, odourless and without taste (Wang et al., 2010).
Tablet manufacturing includes the tabletting operation and post-compaction processes such as coating (to protect from exposure to light, moisture and oxygen; to improve appearance, to mask taste or to control drug release), packaging, handling, storage and use. The formulation must be designed so that tablets meet a required set of mechanical properties to withstand the above-mentioned conditions. One of the key manufacturing requirements is tensile strength. The standard method to characterise the tensile strength of a tablet is the diametrical compression test described by Fell and Newton (1970). The test was first introduced by Carneiro and Barcellos (1953) as an indirect method to measure the tensile strength of rock and concrete. The analytical framework to determine the tensile stress under which the compact undergoes failure is based on a stress solution by H. R. Hertz in 1883 (Mellor and Hawkes, 1971) developed for a cylindrical disk compressed between two diametrically opposite platens. According to Hertz, the tensile strength of the materials is calculated as: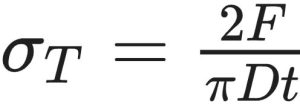
where DT, F, D and t are tensile strength, break force, diameter and thickness of the tablet, respectively. It is intuitive to consider that by increasing the compaction force applied by the punches the density and strength of the resulting compact will increase accordingly.
Various tabletting related concepts are in use, e.g. the United States Pharmacopeia (USP40) issued a supplement in 2017 using following definitions:
- compressibility: solid volume fraction as a function of compaction pressure,
- compactability: tensile strength as a function of solid fraction,
- tabletability: tensile strength as a function of compaction pressure.
Compactability and tabletability relationships have been studied extensively (Balshin, 1949, Ryshkewitch, 1953, Higuchi et al., 1954, Shotton and Ganderton, 1961, Hasselman, 1969, Schiller, 1971, Leuenberger, 1982, Fleck, 1995, LaMarche et al., 2014, Persson and Alderborn, 2018) and empirical or semi-empirical relations were established. A common feature of these equations is that the compactability or tabletability are determined for a given powder mixture. Theories of tensile strength of powder compacts were proposed by Rumpf (1962) and Smalley and Smalley (1964) were the effects of particle size and interparticle forces were considered. There has been an increasing interest to describe and predict the tablet tensile strength of binary mixtures from individual component property contributions (Chan et al., 1983, Bangudu and Pilpel, 1984). The theory of Cheng (1968) accounts for particle size distribution, powder density and interparticle force. More recent research focussed on the rule of mixtures to predict tablet strength from the properties of individual powder constituents (Leuenberger, 1982, Kuentz, 1999, Kuentz and Leuenberger, 2000, Ramirez et al., 2004, Wu et al., 2005, Wu et al., 2006, Busignies et al., 2006, Michrafy et al., 2007, Etzler et al., 2011, Busignies et al., 2012, Juban et al., 2015, Capece et al., 2015, Reynolds et al., 2017, Radojevic and Zavaliangos, 2017).
In this paper we extend the data set produced by Reynolds et al. (2017) with experiments using lubricated mixtures. The methodology developed by Reynolds et al. (2017) is adopted where the rules of mixture is based on the volume fraction of an individual component under the same compaction pressure, as illustrated in Fig. 1. The data analysis methodology is extended to consider the ejection stress and the effect of lubricants. The key contribution of the paper is the concept of Successful Formulation Window (SFW) a predictive analysis tool that determines the porosity range and the compaction pressure range (window) needed to compress a given powder formulation into an acceptable tablet. The SFW addresses the mechanical aspects of formulation and tablet pressing and involves the following steps:
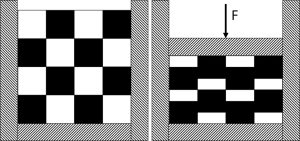
Adapted from Reynolds et al. (2017).
- identify the critical quality attributes that describe the mechanical behaviour of the tablets,
- for each individual powder material in the formulation characterise:
- (a) compressibility,
- (b) compactability,
- (c) ejection stress,
- employ and verify the rules of mixtures to predict the compressibility, compactability and ejection stress of a mixture,
- verify the methodology using experimental data for the formulation,
- data visualization.
4. Materials
The materials considered in this work are common pharmaceutical excipients used in tablet formulations: Microcrystalline cellulose, Calcium Phosphate, Mannitol and Magnesium Stearate (a lubricant). The materials were characterised as single components powders and mixtures. Information of grades, manufacturer and the composition of the formulations studied is presented in Table 4. The physical properties of the powders, including average particle size, specific surface area, bulk density and true density are presented in Table 5.
Microcrystalline cellulose (MCC) is described as a soft, ductile material with high compressibility (Rowe and Roberts, 1995), which undergoes large plastic deformation under pressure without showing brittleness. As plastic deformation takes place the contact areas between particles increases which leads to increased interparticle bonding (compactability). MCC exhibits time dependent behaviour (viscoelasticity) (Doelker, 1993, Bolhuis and Anthony Armstrong, 2006) and it is highly hygroscopic. Due to plastic deformation and low brittleness the particles do not fracture during loading and therefore no new surfaces are created which can lead to lubrication sensitivity (Bos et al., 1991, Zuurman et al., 1999, Hoag et al., 2008, Wang et al., 2010). MCC has a low coefficient of friction and low die wall residual stress compared to Mannitol (Doelker, 1993, Doelker and Massuelle, 2004) which lowers its requirements for lubrication. Detailed information about critical material attributes of MCC applied in direct compaction (DC) can be found in a review paper (Thoorens et al., 2014).
Mannitol is commonly used as a diluent in tablet formulations and behaves similarly to other sugars such as lactose or sucrose. Mannitol undergoes fragmentation under loading, however, also shows plastic deformation (Reynolds et al., 2017), which leads to high compactability. As a result of fragmentation Mannitol is less sensitive to lubrication than MCC. However, Mannitol has a higher coefficient of friction, therefore a lubricant is normally added to the formulation. Mannitol is non-hygroscopic and has good chemical stability.
DiCalcium phosphate (DCPA, ATAB) is typically used in nutritional – health and food industry as a source of calcium and behaves similar to a ceramic powder. DCPA is a brittle material and fragments under pressure (Rowe and Roberts, 1995). Dicalcium phospate is less sensitive to lubrication due to a high particle fragmentation because new clean surfaces are created which are not exposed to lubricant. However, the addition of lubricant decreases flowability and compactability (Hwang and Peck, 2001).
Scanning electron microscopy (SEM) images of bulk powder of MCC, Mann and ATAB are shown in Fig. 2.
Mixtures and lubricated powders. The binary and ternary mixtures of pure excipients are labelled using the mass fraction of the individual components (see Table 4). In addition, two batches of Mannitol lubricated with Magnesium stearate with mass fractions of 0.5% and 1% were characterized. The grade and manufacturer of the Magnesium stearate are listed in Table 4 and the physical properties are presented in Table 5.
Magnesium stearate (MgSt) is a boundary lubricant, categorized as a metallic salt of fatty acids with low melting point and good chemical stability. However due to the manufacturing process Magnesium stearate presents various impurities that can cause incompatibility with APIs. Due to its non-polar molecular structure it is insoluble in water.
Table 4. Grade and source of pure powder materials.
| Excipients | Abbreviated name | Grade | Manufacturer | Mass fraction (%) |
|---|---|---|---|---|
| Microcrystalline cellulose | MCC102 | Avicel Ph102 | FMC Biopolymer, Ireland | – |
| Mannitol | Mann | Pearlitol SD200 | Roquette, France | – |
| DiCalcium Phosphate | ATAB | ATAB | Univar Innophos, USA | – |
| Lubricant | ||||
| Magnesium Stearate | MgSt | MgSt MF2-V | Peter Greven | – |
| Binary mixtures | ||||
| MCC102+Mann | M12_51/49 | – | – | 51:49 |
| MCC102+ATAB | M13_35/65 | – | – | 35:65 |
| Mann+ATAB | M23_34/66 | – | – | 34:66 |
| Ternary mixtures | ||||
| MCC102+Mann+ATAB | M123_26/25/48 | – | – | 26:25:48 |
| MCC102+Mann+ATAB | M123_15/58/27 | – | – | 15:58:27 |
| MCC102+Mann+ATAB | M123_11/10/79 | – | – | 11:10:79 |
| MCC102+Mann+ATAB | M123_59/14/27 | – | – | 59:14:27 |
| Lubricated material | ||||
| Mann+MgSt | Mann_05% | – | – | 99.5:0.5 |
| Mann+MgSt | Mann_1% | – | – | 99:1 |
Download the full article as PDF here Successful Formulation Window for the design of pharmaceutical tablets with required mechanical properties
or read it here
P. Polak, I.C. Sinka, G.K. Reynolds, R.J. Roberts, Successful Formulation Window for the design of pharmaceutical tablets with required mechanical properties, International Journal of Pharmaceutics, 2023, 123705, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.123705.

