Investigating the Effectiveness of Different Porous Nanoparticles as Drug Carriers for Retaining the Photostability of Pinosylvin Derivative
Abstract
Pinosylvin monomethyl ether (PsMME) is a natural compound known for its valuable bioactive properties, including antioxidant and anti-inflammatory effects. However, PsMME’s susceptibility to photodegradation upon exposure to ultraviolet (UV) radiation poses a significant limitation to its applications in the pharmaceutical field. This study, for the first time, introduces a strategy to enhance the photostability of PsMME by employing various nanoformulations. We utilized mesoporous silica nanoparticles (MSNs) coated with polydopamine via a poly(ethylene imine) layer (PDA–PEI–MSNs), thermally carbonized porous silicon nanoparticles (TCPSi), and pure mesoporous polydopamine nanoparticles (MPDA). All these nanocarriers exhibit unique characteristics, including the potential for shielding the drug from UV light, which makes them promising for enhancing the photostability of loaded drugs. Here, these three nanoparticles were synthesized and their morphological and physicochemical properties, including size and ζ-potential, were characterized. They were subsequently loaded with PsMME, and the release profiles and kinetics of all three nanoformulations were determined. To assess their photoprotection ability, we employed gas chromatography with a flame ionization detector (GC-FID) and gas chromatography–mass spectrometry (GC-MS) to assess the recovery percentage of loaded PsMME before and after UV exposure for each nanoformulation. Our findings reveal that MPDA exhibits the highest protection ability, with a remarkable 90% protection against UV light on average. This positions MPDA as an ideal carrier for PsMME, and by extension, potentially for other photolabile drugs as well. As a final confirmation of its suitability as a drug nanocarrier, we conducted cytotoxicity evaluations of PsMME-loaded MPDA, demonstrating dose-dependent drug toxicity for this formulation.
Introduction
Pinosylvin and its derivatives are naturally occurring stilbenoid compounds found in spruce and pine trees, and have garnered considerable attention due to their diverse range of biological and pharmaceutical activities, encompassing antimicrobial, anti-inflammatory, anticancer, neuroprotective, and anti-allergic effects [1]. While the anti-inflammatory impact of stilbenoid compounds bears resemblance to that of established commercial anti-inflammatory agents [2], their physicochemical properties limit their therapeutic potential. PsMME and stilbenoids, in general, typically feature long hydrocarbon chains.
As a rule, compounds with such hydrocarbon chains often exhibit low solubility in water [3]. Compromised aqueous solubility and the resulting poor bioavailability, coupled with heightened susceptibility to external influences, such as air, UV light, and oxidative enzymes, present substantial challenges [3]. UV light exposure, in particular, triggers polymerization and cis–trans isomerization of stilbenoids [4], thus diminishing their stability and efficacy. To address these limitations and unlock the full therapeutic capabilities of stilbenoids, there is a crucial need to tailor the physicochemical attributes of these compounds. This endeavor is aimed at maintaining therapeutic doses of stilbenoids at a safe level, by simultaneously ensuring their high stability and suitable solubility.
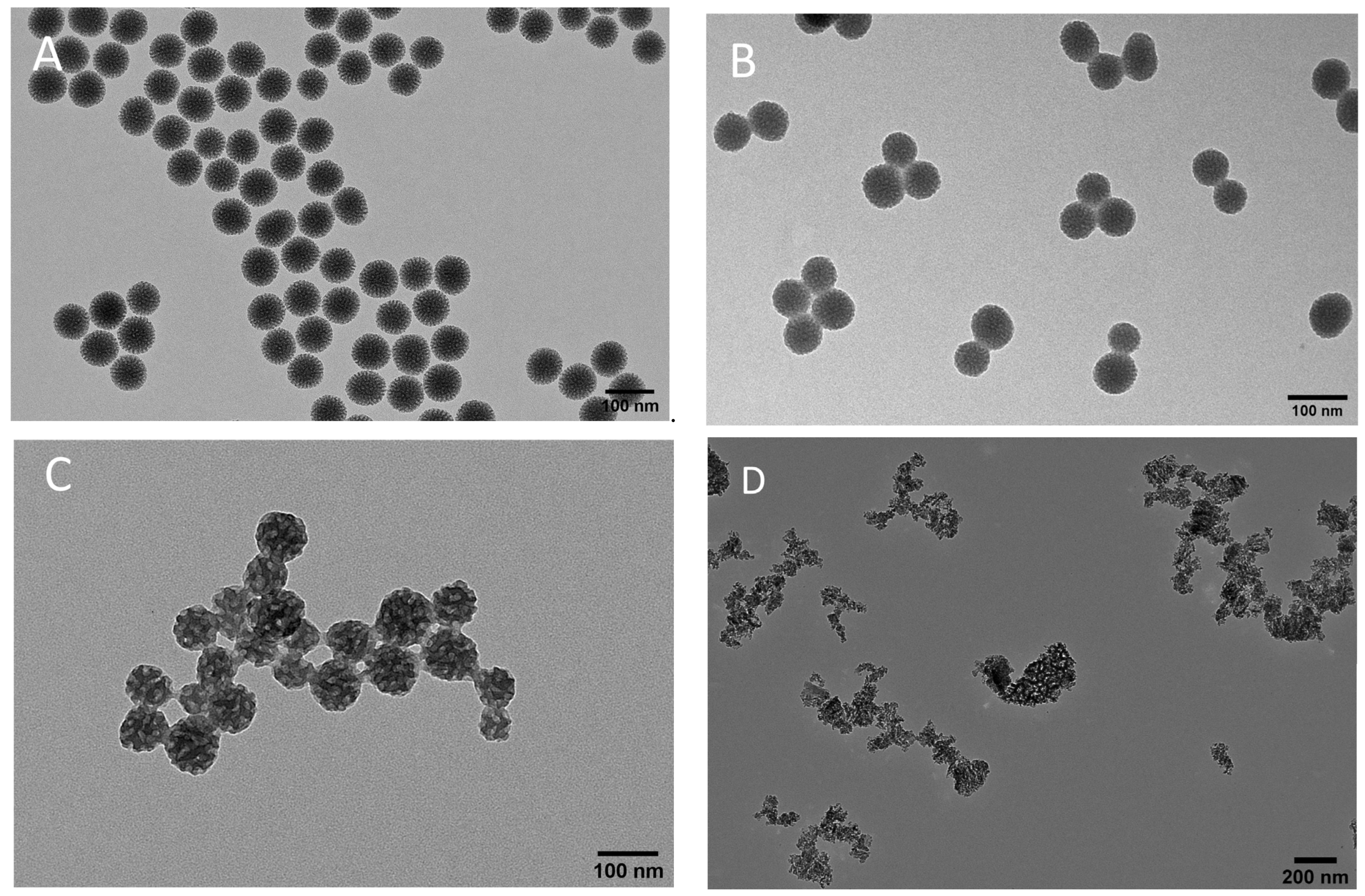
A recent study has indicated that enabling formulations, especially through the encorporation of stilbenoids, can augment their therapeutic outcomes [5]. Therefore, our study utilizes versatile nanocarriers: three different types of mesoporous nanoparticles, to modulate the physicochemical properties of pinosylvin monomethyl ether (PsMME). Mesoporous silica nanoparticles (MSNs) have long been used as carriers to effectively increase the solubility and dissolution rates of diverse drugs and biologically active agents [6]. Furthermore, the inorganic silica matrix of MSNs offers a platform for stabilizing the incorporated agents during storage, thereby extending the shelf life of the loaded drug [7]. Given that amorphous silica is optically transparent, an effective approach to enhance the photostability of incorporated agents involves coating MSNs with polydopamine (PDA), a well-known UV-shielding material that helps preserve the biological activity of photolabile substances [8]. As a follow-up to this system, mesoporous polydopamine (MPDA) nanoparticles composed only of PDA, i.e., without the MSN skeleton, have also gained prominence, leveraging the simplicity and biocompatibility of PDA [9]. However, the properties of this system are not as flexibly controllable as in the case of MSNs.
Moreover, porous silicon (PSi) materials, also known for their stabilizing matrix, biocompatibility, biodegradability, and enhanced drug permeability properties, have emerged as valuable tools for a variety of biomedical applications [10]. Distinguished from the bottom-up fabrication method of mesoporous silica materials, PSi materials are created through top-down etching and milling techniques. Their distinct optical properties, including the ability to absorb UV-light across various wavelengths due to their unique porous structure, position them as ideal candidates for further exploration as carriers of photolabile drug compounds [11,12]. The surface chemistry of PSi is also easily modifiable, such as through thermal carbonization with acetylene (TCPSi), providing the nanocarrier with high aqueous stability [13] (contrary to their silica counterparts).
Introduction of dark color components into nano-systems has been documented to enhance the saturation of structural colors by effectively absorbing scattered light across a broad wavelength spectrum [14]. In the current study, we conducted a comprehensive evaluation of three distinct dark nanoparticles, namely PDA–PEI–MSNs [PEI = poly(ethylene imine)], MPDA, and TCPSi, with the aim of exploring their potential as nanocarriers for augmenting the photostability of PsMME as model drug. First, by employing techniques such as dynamic light scattering (DLS) and transmission electron microscopy (TEM), we characterized the physicochemical attributes of the nanoformulations. Subsequently, we assessed their drug loading degree (DL%), drug release profiles, and photostability, allowing for a systematic comparison of the nanoparticles’ respective performances. Notably, due to the superior photostability-conveying properties exhibited by MPDA, these nanoparticles were also assessed for their cytotoxicity with and without loaded drug to determine their cytocompatibility as a basis for further studies.
In summary, this study sets the stage for a detailed exploration into the potential of tailored nanocarriers to enhance the therapeutic viability of PsMME and other photolabile drugs, marking a significant stride toward overcoming its inherent limitations and maximizing its pharmaceutical potential.
Download the full article as PDF: Investigating the Effectiveness of Different Porous Nanoparticles as Drug Carriers for Retaining the Photostability of Pinosylvin Derivative
or read it here
Howaili, F.; Saadabadi, A.; Mäkilä, E.; Korotkova, E.; Eklund, P.C.; Salo-Ahen, O.M.H.; Rosenholm, J.M. Investigating the Effectiveness of Different Porous Nanoparticles as Drug Carriers for Retaining the Photostability of Pinosylvin Derivative. Pharmaceutics 2024, 16, 276. https://doi.org/10.3390/pharmaceutics16020276
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


