European CDMOs capability mapping – Small Molecules and Biologics

Fuliginous Management Ltd is a service providing company to the Contract Manufacturing Market, supporting both CMOs and Pharma Companies cooperating with CMOs. Get prepared for 2021 and acquire the updated CDMOs capibility mapping.
Fuliginous Management Consulting offers services to the Pharma CDMO industry, supporting:
- CDMOs willing to take their business to the next level by applying tailor-made best in class practices for sustainable and profitable growth.
- Pharma Companies willing to review and optimize their external manufacturing network for efficient risk and cost management, aligned with company’s strategy.
- Both CDMOs and Pharma Companies by liaising Customer and Supplier with best match, based on a specific project.
- Investors to the CDMO market by evaluating current and future Target perspectives and defining what needs to be done (and how) to achieve a challenging but realistic business plan.
Our extended experience and market knowledge as well as being exposed to different business models allow us to provide proposals / solutions tailor-made to the needs of each customer. Our deliverables are specific, avoid generalities and wishful thinking and provide solutions that work.
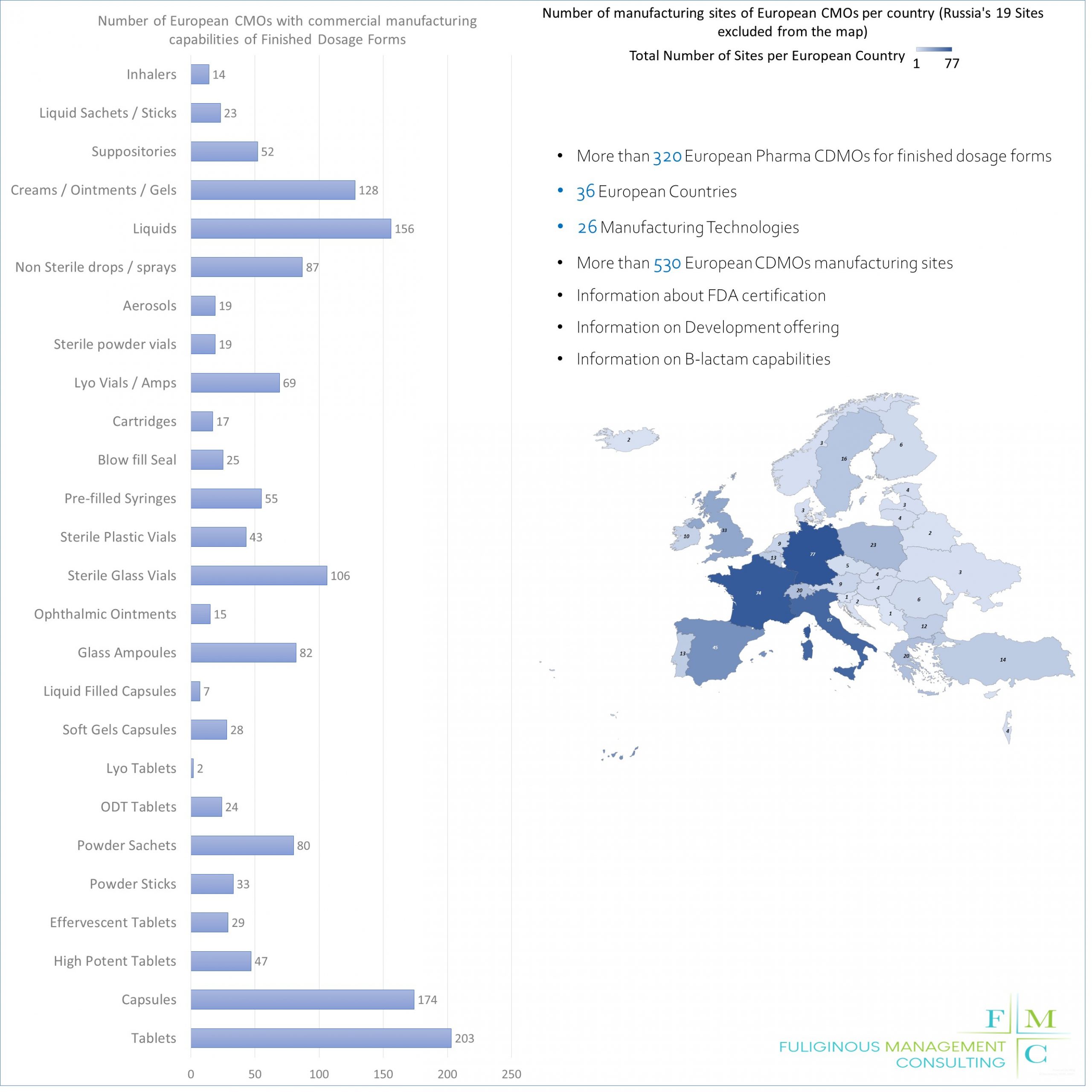
We believe that a key ingredient for success for our customers is to understand better the business environment they operate. In this direction we have developed a European CMO capability mapping including 25 manufacturing technologies for more than 250 CMOs operating in Europe. This mapping can support Pharma companies outsourcing their production, to have a detailed overview of the European CMO market and the detailed capabilities of each player.
The European Pharmaceutical CMOs database contains CMOs (or companies which offer CMO services to third parties) that focus on the production of pharmaceutical finished products and have the capability to produce commercial scale batches. As European CMOs we define companies that have at least one site able to produce pharmaceutical dosage forms for commercial productions in Europe. Overall it includes more than 320 CMOs and more than 550 manufacturing sites globally out of which around 530 are located in Europe. The overall number of European countries with manufacturing sites is 36 including Russia, Turkey and Israel (although not in a significant amount)
It also includes:
- The number of manufacturing sites of each CMO (excluding sites that do not manufacture finished dosage forms). So for example if one CMO has 3 sites overall and one of them carries out API production, this site is excluded from the number that we include and the number of sites shown in the database is 2
- The location of the manufacturing sites so that you can know how many sites they have in each country
- 26 different technologies (the ones that you see in the picture attached)
- Information for FDA approved sites. When a CMO has an FDA approved site, we include this information but this does not necessarily mean that the site is located in Europe (although in the majority of cases, this is the case)
- Beta lactams capability is also included but not in detail
- For information purposes the database includes the information about the capability of the CMOs to produce food supplements, cosmetics and VET products but we have not included further details
- Webpages of each CMO as well as a contact email. In certain cases, there is no available contact email because CMOs use an online contact form and no email is shared
Biologics CDMOs Mapping
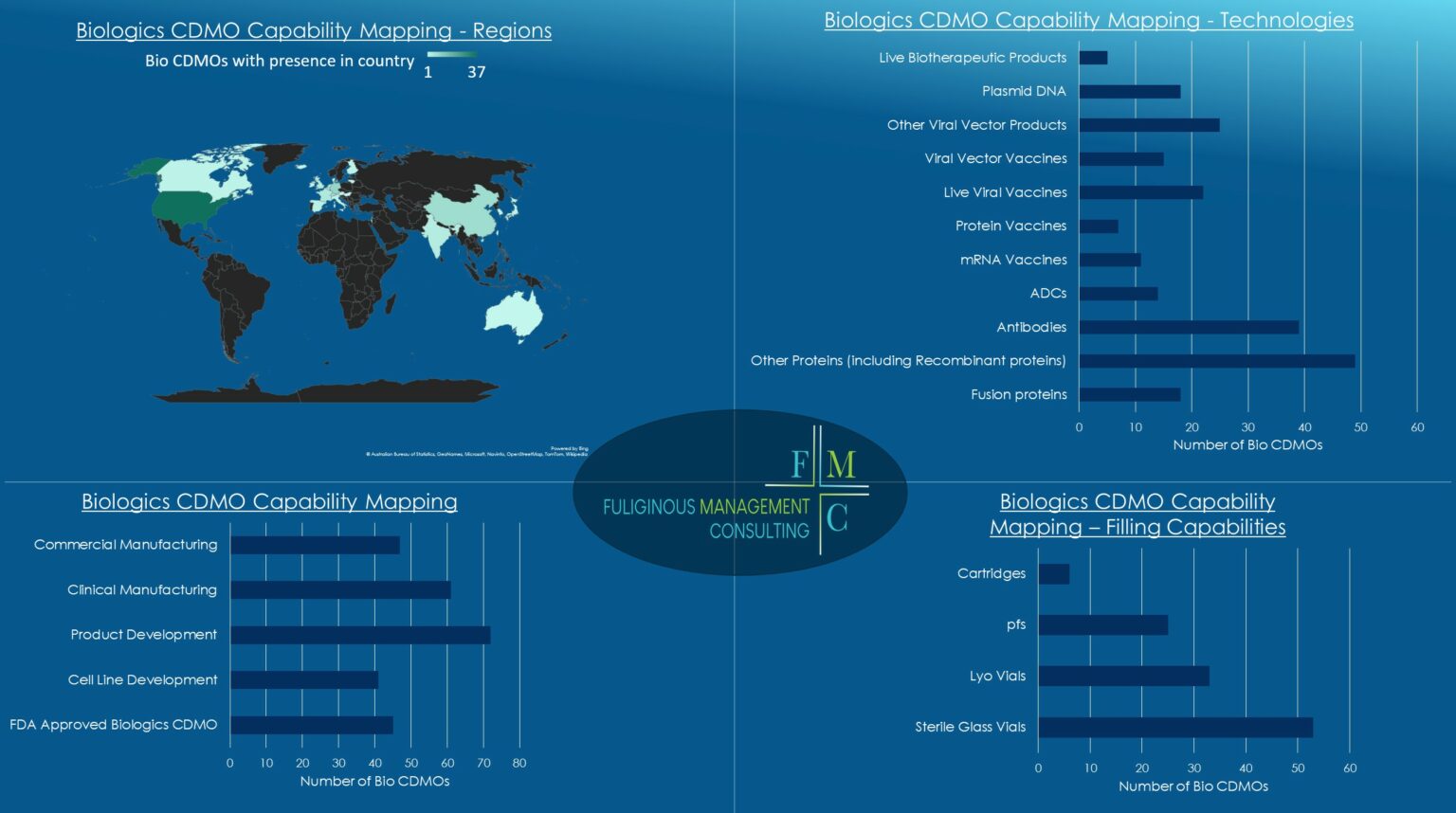 Apart from the fact that there are many different types of biologic products, at the moment there are not too many CDMOs able to manufacture drug substance and then produce a product, let alone that there are some CDMOs that don’t have commercial scale capabilities and some that do not have even filling capabilities (and if they have filling capabilities, not all of them can fill every type of primary packaging).
Apart from the fact that there are many different types of biologic products, at the moment there are not too many CDMOs able to manufacture drug substance and then produce a product, let alone that there are some CDMOs that don’t have commercial scale capabilities and some that do not have even filling capabilities (and if they have filling capabilities, not all of them can fill every type of primary packaging).
So, what we wanted to clarify for us and for our customers, is which CDMO is able to do what and up to what step. In this attempt we checked the profiles of around 70 Bio CDMOs and we checked 11 different types of bio products as well as the main primary packaging (filling) options. Moreover, we mapped which CDMOs offer cell line development, which can offer commercial batches and which are FDA approved.
More information: www.fuliginous.eu or https://www.linkedin.com/company/fuliginous-management-ltd/