The Guide to Formulating Oral Extended-Release Tablets

Extended-release tablets, also known as sustained-release, controlled-release, or time-release tablets, have become increasingly important in the healthcare sector due to their ability to maintain consistent drug levels in the body over a specific period. This drug delivery system provides various benefits, including enhanced patient compliance, minimized side effects, and improved therapeutic efficacy.
Definition of Extended-Release Tablets
Extended-release tablets are medications designed to release an active ingredient over a specific duration gradually. Instead of being immediately released and absorbed once ingested, the drug in these tablets is distributed steadily in the body, ensuring a prolonged therapeutic effect. The goal is maintaining a constant drug concentration in the bloodstream, potentially reducing the dosage frequency and minimizing peaks and troughs in plasma drug levels (Figure 1).
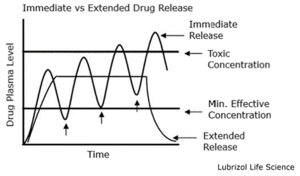
Importance and Benefits of Extended-Release Tablets
The development and use of extended-release tablets are critical in modern medicine. These formulations provide several advantages that improve patient care:
- Improved patient compliance: Patients often need extended-release tablets to take their medication less frequently than conventional immediate-release medications. This can improve adherence to treatment regimens, particularly in chronic conditions requiring long-term medication use.
- Reduced side effects: By maintaining steady drug levels in the body, extended-release tablets can minimize the risk of side effects often associated with high peaks in drug concentration.
- Enhanced therapeutic effect: Extended-release tablets ensure a consistent medication delivery over time, which can enhance the overall effectiveness of the treatment by ensuring maximum absorption and extending the duration of action.
The following sections will delve deeper into the mechanics, development, applications, and patient considerations associated with extended-release tablets. This complete guide aims to provide an in-depth understanding of this crucial aspect of pharmaceutical science and its significant impact on healthcare outcomes.
Basics of Extended-Release Tablets
Mechanism of Action
The primary objective of extended-release tablets is to control the rate at which the drug is released into the system after ingestion. Various techniques, including matrix systems, encapsulation, and coating methods, achieve this. In all cases, the drug is embedded into a system that slowly dissolves or breaks down in the body, allowing a steady release of the medication. The specific mechanism of action varies depending on the type and design of the tablet.
Different Types of Extended-Release Tablets
Extended-release tablets can be broadly categorized into two types:
- Diffusion-Controlled Systems: These include reservoir devices and matrix devices. In reservoir devices, the drug core is surrounded by a polymer membrane that controls the drug release rate. In matrix devices, the drug is distributed throughout an insoluble polymer matrix, and the drug diffusion rate governs its release (along with matrix erosion at later time points).
- Dissolution-Controlled Systems: These systems control drug release by modifying the rate of dissolution of the drug in the gastrointestinal tract. This is usually achieved through special coatings or the incorporation of the drug in a water-soluble matrix.
Common Examples of Extended-Release Medications
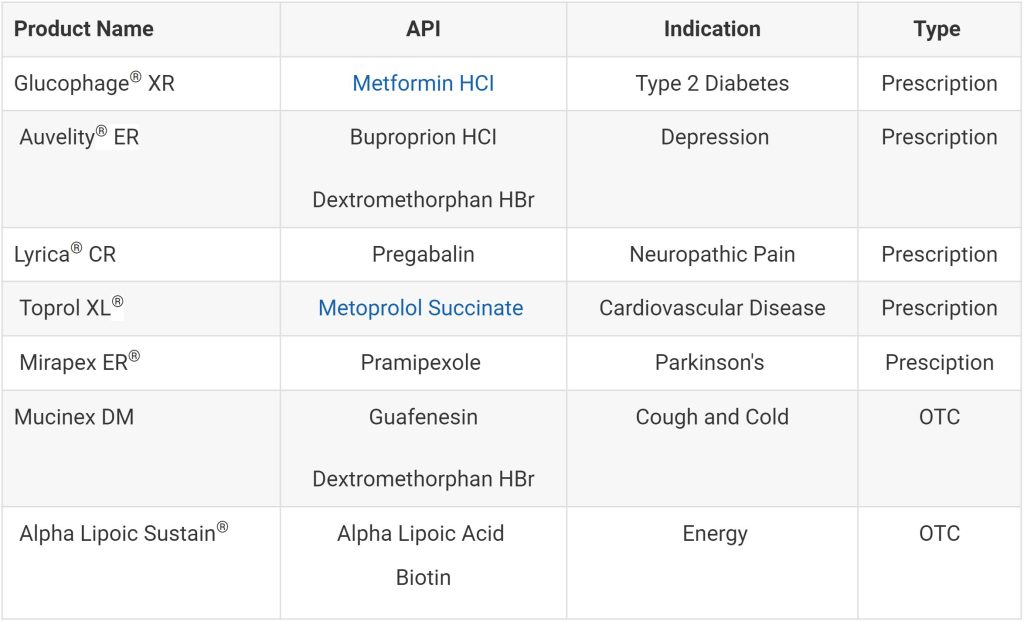
Many medications come in extended-release formulations. Some common examples include certain cardiovascular drugs (e.g., metoprolol succinate), pain relievers (e.g., pregabalin CR), antidiabetic drugs (e.g., metformin ER), and psychiatric medications (e.g., dextromethorphan/bupropion ER).
Table 1: Common Examples of Extended-Release Tablets
Development and Manufacturing of Extended-Release Tablets
Pharmaceutical Formulation Techniques
The formulation of extended-release tablets involves a variety of pharmaceutical techniques. The choice of technique largely depends on the properties of the drug, the desired release profile, and the intended route of administration. Some commonly used techniques include:
- Matrix Formulations: The most common approach to achieving oral extended-release, in which drug is mixed with a polymer matrix and compressed into a tablet. The tablet is formulated to release the drug at a controlled rate as the matrix slowly dissolves or erodes in the body.
- Coating Techniques: These involve applying a thin polymer layer to the drug particles or tablets. The coating material and thickness are selected to achieve the desired release rate.
- Encapsulation Techniques: The drug is enclosed within a capsule that dissolves at a controlled rate, releasing the drug over time.
Manufacturing of Extended-Release Tablets
There are several ways to manufacture extended-release tablets, namely:
- Wet Granulation: a process by which dry powders are agglomerated in the presence of a granulating fluid. The mixture is then dried, leaving behind granules of API and excipient(s). These granules are blended with additional ingredients, such as fillers and binders, and compressed into tablets.
- Dry Granulation: a process by which powder mixtures are compressed into granules without the use of liquid. These granules are then blended with additional ingredients, such as fillers and binders, and compressed into tablets.
- Direct Compression: a streamlined process by which powder mixtures of API and specialized excipients are weighed, blended, and compressed into tablets.
In recent years, direct compression has gained traction as an alternative to traditional granulation processes due to its many economic- and resource-based benefits, including:
- Minimized processing steps
- Reduced energy use
- Decreased labor costs
- Higher throughput
Quality assurance and control are critical in manufacturing extended-release tablets to ensure that they meet the desired specifications and release profiles. This involves rigorous testing of the raw materials, in-process materials, and finished products using methods such as dissolution testing, hardness testing, and friability testing.
Regulatory Aspects in the Production of Extended-Release Tablets
The production of extended-release tablets is subject to stringent regulatory requirements to ensure safety, efficacy, and quality. These requirements include Good Manufacturing Practices (GMPs), stability testing, bioequivalence studies, and appropriate labeling.
When selecting excipients an extended-release formulation, it is important to reference the FDA’s Inactive Ingredient Database (IID), which lists excipients found in approved drug products. The IID lists the highest amount of the excipient per unit dose in each dosage form in which it is used. This database is often used by manufacturers when developing new drugs and generic medications because it provides data on inactive ingredients that the FDA has previously vetted.
Pharmacokinetics and Pharmacodynamics of Extended-Release Tablets
How the Body Processes Extended-Release Medications
Pharmacokinetics is how the body absorbs, distributes, metabolizes, and excretes a drug. In the case of extended-release tablets, the drug is typically absorbed in the gastrointestinal tract over a prolonged period. This slow absorption process, combined with subsequent distribution, metabolism, and excretion, contributes to maintaining steady levels of the drug in the body.
Effects of Extended-Release Medications on the Body
Pharmacodynamics deals with the effects of a drug on the body. Extended-release tablets are designed to provide a steady, therapeutic effect over time. By maintaining consistent drug levels, these tablets can ensure that the drug continues to exert its desired effect without causing peaks that may lead to side effects or troughs that might render the medication less effective.
Factors Influencing the Absorption and Distribution of Extended-Release Tablets
Several factors can influence the absorption and distribution of extended-release tablets. These include the patient’s age, body weight, genetic factors, overall health status, the presence of food in the stomach, the pH of the gastrointestinal tract, and the use of other medications. Some APIs may also have properties that introduce challenges, such as poor absorption outside the stomach/upper gastrointestinal tract or short half-lives. These drugs often require a combination of extended-release technology with another approach, such as gastroretention, to extend the absorption window.
Read the original article by Lubrizol
Source: Lubrizol, website https://www.lubrizol.com/Health/Blog/2023/08/The-Guide-to-Oral-Extended-Release-Tablets, Posted by Nicholas DiFranco on 08/30/2023

