Biosafety of mesoporous silica nanoparticles; towards clinical translation
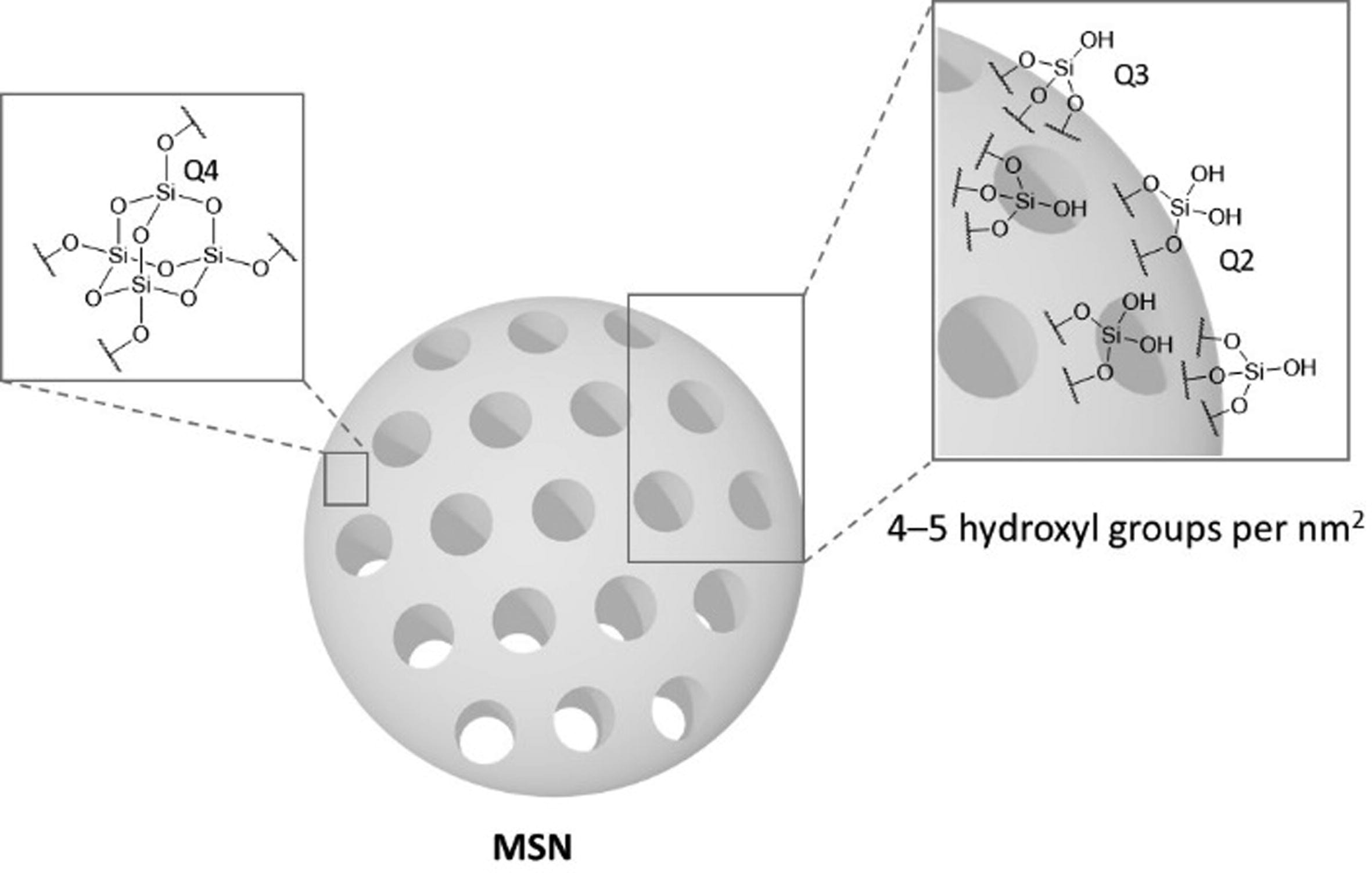
Mesoporous silica nanoparticles (MSNs) have attracted the attention of chemists, who have developed numerous systems for the encapsulation of a plethora of molecules, allowing the use of mesoporous silica nanoparticles for biomedical applications. MSNs have been extensively studied for their use in nanomedicine, in applications such as drug delivery, diagnosis, and bioimaging, demonstrating significant in vivo efficacy in different preclinical models. Nevertheless, for the transition of MSNs into clinical trials, it is imperative to understand the characteristics that make MSNs effective and safe. The biosafety properties of MSNs in vivo are greatly influenced by their physicochemical characteristics such as particle shape, size, surface modification, and silica framework. In this review, we compile the most relevant and recent progress in the literature up to the present by analyzing the contributions on biodistribution, biodegradability, and clearance of MSNs. Furthermore, the ongoing clinical trials and the potential challenges related to the administration of silica materials for advanced therapeutics are discussed. This approach aims to provide a solid overview of the state-of-the-art in this field and to encourage the translation of MSNs to the clinic.
1. Introduction
The application of nanoparticles as a promising technology in the biomedical field, referred to as nanomedicine, has attracted the attention of chemists in the last decades. [1] Particularly, mesoporous silica nanoparticles (MSNs) have gained attention since their first synthesis in the early 1990s[2] and their first use in drug delivery in 2001 reported by Vallet-Regi at al.[3] MSNs have unique properties such as a confined space to encapsulate therapeutic compounds and attracted the attention of numerous groups that developed drug delivery systems based on MSNs.[4], [5], [6] Besides, MSNs have remarkable characteristics such as adjustable porous structures, tunable and narrow pore size distributions, high pore volumes, high loading capacity, and high silanol density that allows easy dual-functionalization (exterior and interior).[7], [8], [9] Further report in the 2000s of the first gated MSNs[10] and the possibility to develop gated MSNs operative in aqueous solution[11] fueled the development of numerous materials containing molecular gates (also known as gatekeepers or nanovalves) sensitive to different stimuli. These molecular gates are grafted onto the external surface of the MSN blocking cargo transport from the pores to the solution, that is released on-command in response to a certain stimulus (chemical, biochemical or physical) that usually breaks apart the molecular gate, opening the pore for cargo release.[7], [12], [13] As a result, the functionalization of MSNs with gatekeepers allows obtaining efficient drug delivery carriers improving the biocompatibility, cell membrane penetration, and site-specific delivery and controlled release of drugs and therapeutic agents.[7], [14], [15], [16], [17]

Gated MSNs have demonstrated a wide range of biomedical applications, including encapsulation and delivery of drugs, proteins, or genes for in vivo biological imaging, and therapies such as photothermal therapy, photodynamic therapy, radiotherapy, ultrasound therapy, anti-bacterial applications, and tissue engineering.[18] Moreover, gated materials have also been used in sensing and communication applications.[19], [20], [21], [22] Gated MSNs for drug delivery have been used successfully in in vivo models, resulting in an improvement of the solubility of the encapsulated drugs and in a reduction of undesired side effects. [23], [24], [25], [26], [27], [28] From a chemical point of view, nanomaterials present several properties that can be tailored to achieve specific applications. Consequently, many MSNs-based nanoparticles with different compositions, structures, morphologies, and functionalizations have been synthesized with excellent results in terms of cytotoxicity, therapeutic effect, and compatibilities. An exhaustive revision of synthetic protocols of MSNs has been published by Croissant and coworkers.[29]
Although the application of MSNs to clinical medicine seems feasible based on many promising in vitro results obtained to date, the in vivo translation remains challenging as the administration may exhibit different results under physiological environments and might have adverse effects leading to long-term safety issues. In addition, the effect of opsonization, enhanced permeability, and retention (EPR) effect, or the transportation in the blood stream are difficult to replicate in in vitro systems. Moreover, the cytotoxicity of MSNs captured by the reticuloendothelial system (RES) organs is rarely studied in in vivo models. Therefore, this field raises many new questions regarding the pharmacokinetic and safety behavior of nanoparticles within living systems.
Compared to the number of preclinical studies, only a few silica-based nanomaterials have been FDA authorized for clinical trials. To reach clinical permission, the application of nanotechnology requires synthesizing nanoparticles with optimal in vivo features, not only for therapeutics outcomes but also for bio-safety concerns. Nevertheless, the literature around the biocompatibility, biodistribution, and biodegradation of MSNs is inconsistent, revealing an important gap in the complex knowledge of nanomedicine. Besides, the biological outcome of MSNs depends significantly on the nanoparticles’ physicochemical properties as it also happens with other nanoparticles.[30], [31]
Despite many excellent articles and reviews have been previously published related to MSNs in biomedical applications,[32], [33], [34], [35], [36], [37], [38] the lack of evidence in the literature regarding safety issues of MSNs during in vivo studies has emerged as the most critical barrier to clinical translation. In this review, we have compiled selected examples in the literature up to the present, with an emphasis on the biodistribution, biodegradability, and clearance of MSNs showing the potential and challenges related to the administration of MSNs for advanced therapeutics. Along the review, we also evaluate the main factors influencing the MSNs fate when administered in living organisms and highlight the strategies to reduce potential toxicities from MSNs administration.[30], [31] This approach aims to provide a general conceptual view of this field and aims to help researchers to develop MSNs to reach the clinical application.
Download the full study as PDF here: Biosafety of mesoporous silica nanoparticles; towards clinical translation
or read it here
Araceli Lérida-Viso, Alejandra Estepa-Fernández, Alba García-Fernández, Vicente Martí-Centelles, Ramón Martínez-Máñez, Biosafety of mesoporous silica nanoparticles; towards clinical translation, Advanced Drug Delivery Reviews, 2023, 115049, ISSN 0169-409X,
https://doi.org/10.1016/j.addr.2023.115049.

