N-nitrosamine Mitigation with Nitrite Scavengers in Oral Pharmaceutical Drug Products
N-nitrosamines are likely human carcinogens. After N-nitrosamine contaminants were detected in pharmaceutical products in 2018, regulatory authorities set a framework for the risk assessment, testing and mitigation of N-nitrosamines in drug products. One strategy to inhibit the formation of N-nitrosamines during the manufacture and storage of drug products involves the incorporation of nitrite scavengers in the formulation. Diverse molecules have been tested in screening studies including the antioxidant vitamins ascorbic acid and α-tocopherol, amino acids, and other antioxidants used in foods or drugs, for inclusion into drug products to mitigate N-nitrosamine formation. This review article outlines key considerations for the inclusion of nitrite scavengers in oral drug product formulations.
Introduction
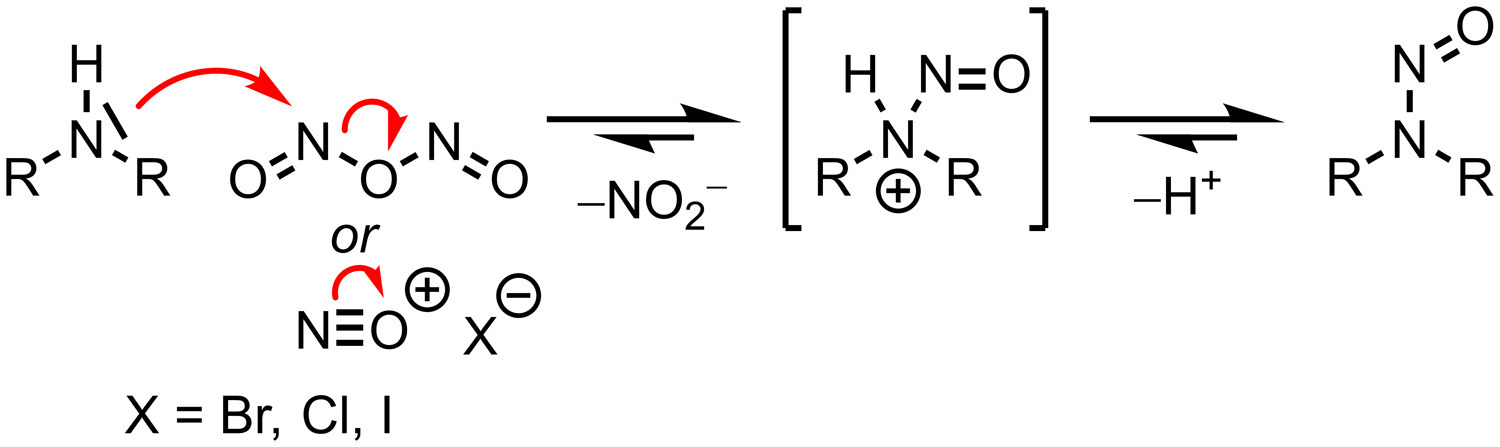
N-nitrosamines are organic compounds that can be found in the environment, diet, and are also produced endogenously.1, 2, 3, 4 A number of N-nitrosamines are carcinogenic and can be present as contaminants in drug products.5,6 They are formed by the reaction of secondary or tertiary amines together with nitrosating agents such as nitrite salts under acidic conditions.7,8 Secondary and tertiary amines may be present in the drug product as the active ingredient, its degradation products, or impurities from chemical synthesis or excipients. Nitrosation reactions can be mitigated in foods through the addition of substances such as ascorbic acid, α-tocopherol and sulfur dioxide.9 Similarly, recent work has proven that such attenuation can also be achieved in pharmaceutical products by the addition of excipients capable of nitrite scavenging.10, 11, 12
N-nitrosamines are part of the so-called “cohort of concern” impurities in pharmaceutical products, and some are considered “possible” or “probable” human carcinogens by the International Agency for Research on Cancer.8,13 Representative members of the N-nitrosamine class have been found to cause cancer in at least 40 different animal models.4,13 Even so, the carcinogenicity of most N-nitrosamines has not been tested experimentally and there remains considerable uncertainty about the exact role of N-nitrosamine formation on cancer incidence in humans.2,14
N-nitrosamines require metabolic activation by oxidative reactions to impart their carcinogenic effect.15 Metabolic activation occurs either via a single α-hydroxylation resulting in fragmentation into a diazonium hydroxide and an aldehyde, or via consecutive α-hydroxylations forming unstable nitrosamides. Both these processes ultimately result in the formation of carbocations or diazonium cations that are highly reactive and capable of alkylating macromolecules and higher-level structures such as DNA or RNA.16
After N-nitrosamines were found in angiotensin II receptor blocker (ARB) drug products in 2018, a global investigation into N-nitrosamine contamination of these drug products by regulatory bodies was triggered.17 Initially, ARBs (sartans) were in the focus of the regulatory bodies. Subsequently, and after N-nitrosamine impurities were found in other classes of human medicines such as ranitidine and metformin, the focus of the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Pharmaceuticals and Medical Devices Agency (PMDA) was broadened. The regulatory requirements to perform risk assessments to determine potential N-nitrosamine formation and propose mitigation strategies where applicable were accordingly introduced for all drug products.14,18 While the addition of agents to mitigate N-nitrosamine formation was not specifically mentioned in early guidance documents,8,19 the potential for inhibitors as excipients has been later put forward as a strategy to be considered in the overall management of N-nitrosamine contamination in drug products.20
The addition of nitrite scavenging excipients to formulations was identified and recently tested as a mechanism of action to prevent N-nitrosamine formation in drug products.11,12 As part of compound screening, 19 structurally diverse compounds with an acceptable toxicological profile were evaluated by Homšak et al.11 These included certain amino acids (L-cysteine, histidine, lysine), general antioxidants (propyl gallate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT)), ammonia-related compounds (urea, ammonium sulfate and ammonium chloride), the pyrone maltol, para-aminobenzoic acid (PABA), and vitamins with antioxidant properties (ascorbic acid, α-tocopherol). Likewise, the use of ascorbic acid and α-tocopherol to inhibit N-nitrosamine formation in pharmaceutical products has been specifically mentioned by the FDA.20 Formulations that include a nitrite scavenger could reduce the overall risk of N-nitrosamine formation and keep concentrations within acceptable limits. In this review, we discuss current knowledge and research gaps in the use of nitrite scavengers for N-nitrosamine mitigation, particularly in solid drug products.
Nitrite Scavengers
| Substance | Oral forms on FDA Inactive Ingredient list | Fat/water soluble | Reference to inhibition in vitro | Reference to inhibition in tablets |
|---|---|---|---|---|
| α-Tocopherol | Capsule, solution, tablet, suspension | Fat soluble | 44 | 12 |
| Ascorbic acid | Capsule, powder, solution, suspension, syrup, tablet | Water soluble | 32,42 | 11,12 |
| L-cysteine | Capsule, suspension, tablet | Water soluble | 47,48 | 11 |
| Glycine | Capsule, powder, solution, suspension, tablet | Water soluble | 11 | - |
| Arginine | Tablet | Water soluble | 11 | - |
| Lysine | Only intravenous forms available | Water soluble | 11 | - |
| Histidine | Capsule, suspension | Water soluble | 11 | - |
| Caffeic acid | - | Water soluble | 49 | 12 |
| Ferrulic acid | - | Water soluble | 49 | 12 |
| BHA | Capsule, granule, solution, suspension, syrup, tablet | Fat soluble | 11 | - |
| BHT | Capsule, film, solution, tablet | Fat soluble | 11 | - |
| PABA | - | Water soluble | 11 | 11 |
Download the full review as PDF here: N-nitrosamine Mitigation with Nitrite Scavengers in Oral Pharmaceutical Drug Products
or read it here
Anne-Cécile V. Bayne, Zdravka Misic, René T. Stemmler, Marc Wittner, Margarita Frerichs, Julia K. Bird, Ahmed Besheer, N-nitrosamine Mitigation with Nitrite Scavengers in Oral Pharmaceutical Drug Products, Journal of Pharmaceutical Sciences, 2023, ISSN 0022-3549,
https://doi.org/10.1016/j.xphs.2023.03.022.

