Orthogonal Gelations to Synthesize Core–Shell Hydrogels Loaded with Nanoemulsion-Templated Drug Nanoparticles for Versatile Oral Drug Delivery
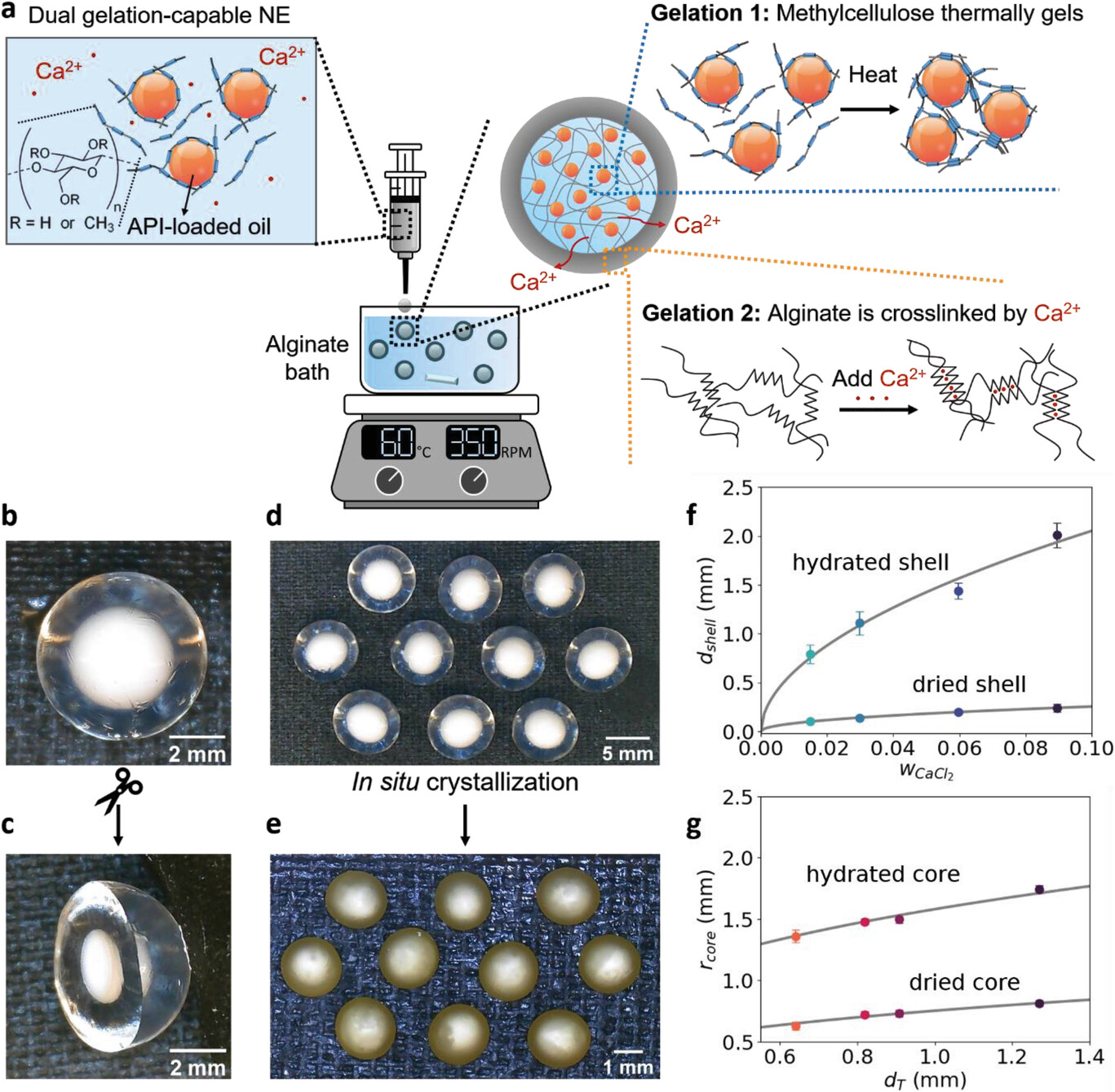
Hydrophobic active pharmaceutical ingredients (APIs) are ubiquitous in the drug development pipeline, but their poor bioavailability often prevents their translation into drug products. Industrial processes to formulate hydrophobic APIs are expensive, difficult to optimize, and not flexible enough to incorporate customizable drug release profiles into drug products. Here, a novel, dual-responsive gelation process that exploits orthogonal thermo-responsive and ion-responsive gelations is introduced. This one-step “dual gelation” synthesizes core–shell (methylcellulose-alginate) hydrogel particles and encapsulates drug-laden nanoemulsions in the hydrogel matrices. In situ crystallization templates drug nanocrystals inside the polymeric core, while a kinetically stable amorphous solid dispersion is templated in the shell. Drug release is explored as a function of particle geometry, and programmable release is demonstrated for various therapeutic applications including delayed pulsatile release and sequential release of a model fixed-dose combination drug product of ibuprofen and fenofibrate. Independent control over drug loading between the shell and the core is demonstrated. This formulation approach is shown to be a flexible process to develop drug products with biocompatible materials, facile synthesis, and precise drug release performance. This work suggests and applies a novel method to leverage orthogonal gel chemistries to generate functional core–shell hydrogel particles.
1 Introduction
Aqueous solubility has emerged as a crucial parameter to determine the bioavailability-limited efficacy of small molecule therapeutics. Hydrophobic active pharmaceutical ingredients (APIs) comprise an estimated 90% of candidate small molecules in the pharmaceutical development pipeline and nearly 40% of drug molecules marketed in orally-delivered drug products.[1] However, the poor bioavailability of hydrophobic APIs often prevents their translation into clinical drug products and is one of the primary causes of the failure of orally-delivered drug candidates in clinical trials.[2-5] This poor bioavailability is attributed to the slow dissolution kinetics of hydrophobic APIs in the aqueous environment of the gastrointestinal (GI) tract. Given the clinical attractiveness and higher patient compliance associated with oral delivery, enabling the oral bioavailability of hydrophobic API products has emerged as an area of active interest in the pharmaceutical industry.[6, 7] Academic researchers and the pharmaceutical industry have responded by developing several solubility-enhancing formulation technologies, including milling/nanosizing and amorphous solid dispersions (ASDs), to address the limitations of poor oral bioavailability.[5, 8-12] Milling or nanosizing refers to the solid handling processes that produce drug products by media-milling large API crystals to reduce crystal size.[10, 13, 14] This approach relies on the substantial improvement in solubility and dissolution kinetics exhibited by API crystals prepared on the nanoscale (<1000 nm). While this “top-down” approach has been employed effectively industrially to reduce crystal size, milling is energy-intensive and has been shown to introduce potentially dangerous contaminants into drug products.[2, 15] ASDs, solid dispersions where the active ingredient is dispersed throughout an excipient matrix, have emerged as an alternative formulation technology.[6, 16] However, ASDs can be difficult to design, because they experience physical instabilities including amorphous–amorphous phase separation and recrystallization.[7, 16] Engineering long-term stability in ASDs has long been a product goal in the pharmaceutical sciences but has not been yet fully realized in practice.[17]
Biocompatible hydrogels have been widely utilized in drug product formulation to encapsulate APIs in a polymeric matrix, deliver therapeutics orally, and control drug release.[18, 19] However, since hydrogels are hydrophilic in nature, they are incompatible to directly formulating hydrophobic APIs.[20] Numerous technologies have been developed to overcome this incompatibility and enable hydrogel formulation of hydrophobic APIs. Most notably, the incorporation of API-loaded hydrophobic nanodomains (including micelles, nanoemulsions, and lipid nanoparticles) into the hydrogel matrix has allowed for the development of high drug-loaded hydrogel formulations of hydrophobic APIs.[21-31] These hydrophobic nanodomains impart control over the formation of API nanoparticles in the polymeric matrix. This approach exploits the benefits of crystal size reduction from the bottom-up, avoiding the limitations of milling and ASDs. Additionally, using “smart,” or stimuli-responsive hydrogels as the polymeric scaffold allows for the incorporation of additional functionality into the formulation, which can be exploited to access desired drug product profiles like controlled release, delayed release, targeted delivery, or immunomodulation. Clever hydrogel design can incorporate responsiveness to temperature, pH, ions, magnetic or electric fields, and biological species, among other external stimuli.[32, 33]
Combination products, drug products that contain multiple APIs, have been shown to be therapeutically beneficial for clinical applications including hypertension,[34, 35] diabetes, tuberculosis,[36, 37] and HIV.[38, 39] Specifically, fixed dosage combinations (FDCs), which contain multiple APIs in the same physical tablet or capsule, are clinically valuable to reduce patient pill burden and improve patient compliance.[40] FDCs can also unlock previously inaccessible drug product profiles, with different drug release profiles enabling therapeutic synergies. However, difficulties manufacturing FDCs have limited their clinical translation,[41-43] particularly for APIs that are compatible with different excipients.[43, 44] Researchers have recently used core–shell structures as a drug product geometry that can structure drugs in distinct polymeric layers, control the release of multiple payloads, and engineer stimuli-responsive functionality.[45-50] Core–shell functionality is vital for delayed-release applications, which are important delivery routes to target enteric and colorectal diseases.[51-53] Yet, manufacturing core–shell polymeric structures typically relies on 3D printing, emulsification with an oil or lipid phase, or multi-step gelations, which have various limitations for the formulation of therapeutics for oral drug delivery.[54, 55]
Here, we introduce the design of a novel dual-responsive gelation process based on biocompatible polymeric materials and nanoemulsions that is capable of simultaneous and orthogonal “dual gelations.” These dual gelations rely on the orthogonal chemistries of thermoresponsive and ionotropic gelations and enable the synthesis of core–shell hydrogel particles with distinct and non-interpenetrating polymeric layers. We selected biocompatible and naturally-derived model polymers to demonstrate this approach: methylcellulose (MC) as the thermogelling polymer and alginate as the inotropic model gelator. MC has long been used as an excipient in pharmaceutical and food formulations because of its reversible thermal sol–gel behavior,[56-59] controllable erosion,[26, 27, 60, 61] and tensioactive capabilities.[62-64] Alginate is also an ideal excipient for controlled delivery,[30, 65-67] where its anionic property can protect therapeutics from being released in the gastric environment,[68-71] making alginate an optimal enteric coating.[67, 72-74] Our approach overcomes the challenges of traditional solubility-enhancement approaches and exploits the advantages of “soft” templates, namely hydrogels and nanoemulsions, in the formulation and delivery of small molecule therapeutics. The resulting core–shell particles have tunable geometry that enables programmable drug release. This method requires few unit operations compared to the current state-of-the-art combination product manufacturing methods, while also structuring distinct hydrogel layers. To our knowledge, it is the first dual stimuli-responsive gelation that structures multiple hydrophobic APIs in distinct polymeric layers. The modular nature of this platform suggests that minimal adaptation can generalize this approach for the formulation of small molecule therapeutics for diverse applications in oral delivery, or for use in generating all-aqueous core–shell hydrogel particles for other applications.
Materials
MC (viscosity: 15 cP, molecular weight ≈14 000 g mol−1), sodium alginate (≈39% in guluronic acid blocks, Mw ≈ 100 kDa), FEN, anisole, Tween 80 (polysorbate 80), calcium chloride (CaCl2), ethanol, sodium dodecyl sulfate, anhydrous disodium hydrogen phosphate (Na2HPO4), anhydrous monosodium hydrogen phosphate (NaH2PO4), mineral oil (ρ = 0.838 g mL−1), and Nile red were purchased from Sigma-Aldrich and used without additional purification steps. IBU (99%, ACROS Organics) was purchased from Fisher Scientific and used without additional purification steps.
Download the full research as PDF here: Orthogonal Gelations to Synthesize Core–Shell Hydrogels Loaded with Nanoemulsion-Templated Drug Nanoparticles for Versatile Oral Drug Delivery
or read it here
, , , Orthogonal Gelations to Synthesize Core–Shell Hydrogels Loaded with Nanoemulsion-Templated Drug Nanoparticles for Versatile Oral Drug Delivery. Adv. Healthcare Mater. 2023, 2301667.
https://doi.org/10.1002/adhm.202301667

