Optimizing API Load and Minimizing Tablet Weight Leveraging an Innovative DC Mannitol
Presented at the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical (PBP) Technology, 18 – 21 March 2024, Vienna, Austria:
INTRODUCTION
Tablets with stability issues often use mannitol, a stable, water-soluble excipient compatible with most drugs. Direct compression is a preferred method for its simplicity and cost-effectiveness. However, directly compressible mannitol grades have a high tendency to capping1, especially with increased tableting speed or compression forces. Solutions to overcome capping include precompression steps, reduced production speed, or lower compression force, but often require reducing the API load for satisfactory production yield.
OBJECTIVES
PEARLITOL® 200 GT is an innovative granulated beta mannitol for direct compression which properties have been optimized to improve tabletability and limit the occurrence of capping. It was demonstrated that the high powder density of this new and innovative directly compressible mannitol, among other properties, explains its unique feature of improving tabletability and suppressing tablet capping.2
The main benefit of improved tabletability is the possibility to increase the API load, with two beneficial consequences:
- Increase the API dosage for the same tablet weight.
- Decrease the weight and size of tablet for a given API dosage.
Sertraline was used as model API with poor tabletability. Increasing API loads were tested with and without precompression, using a spray-dried mannitol as reference direct compression (DC) mannitol to quantify the API ratio increase or tablet weight decrease when moving to PEARLITOL® 200 GT.
Materials
Granulated mannitol: PEARLITOL® 200 GT (GTman), spray-dried mannitol PEARLITOL® 200 SD (SDman), both from Roquette Frères (Lestrem, France); Roquette Magnesium Stearate from Roquette Frères (Lestrem, France); Sertraline: StarPharm (China).
RESULTS
The sertraline powder had very poor powder flow properties and was not usable on a rotary tablet press. Blends with SDman were free flowing up to 15% sertraline (see table 1). The blend with 20% sertraline was not free flowing and to avoid inconsistent die feeding, a force feeder was used for all trials on a tablet press simulator.
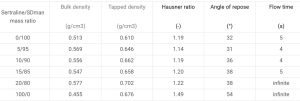
GTman possesses better flow properties than SDman and blends with sertraline were free flowing up to 25% (table 2). Tablets were produced up to 40% sertraline, but an improvement of the flow will be required for an industrial production, for example by adding a glidant.
The tableting study was carried out in two stages: first trials without precompression then trials with precompression. Logically, tableting of powder blends with tendency to capping is done using a precompression step. It was expected that precompression being used to limit the capping will decrease the difference between the two DC mannitol grades.
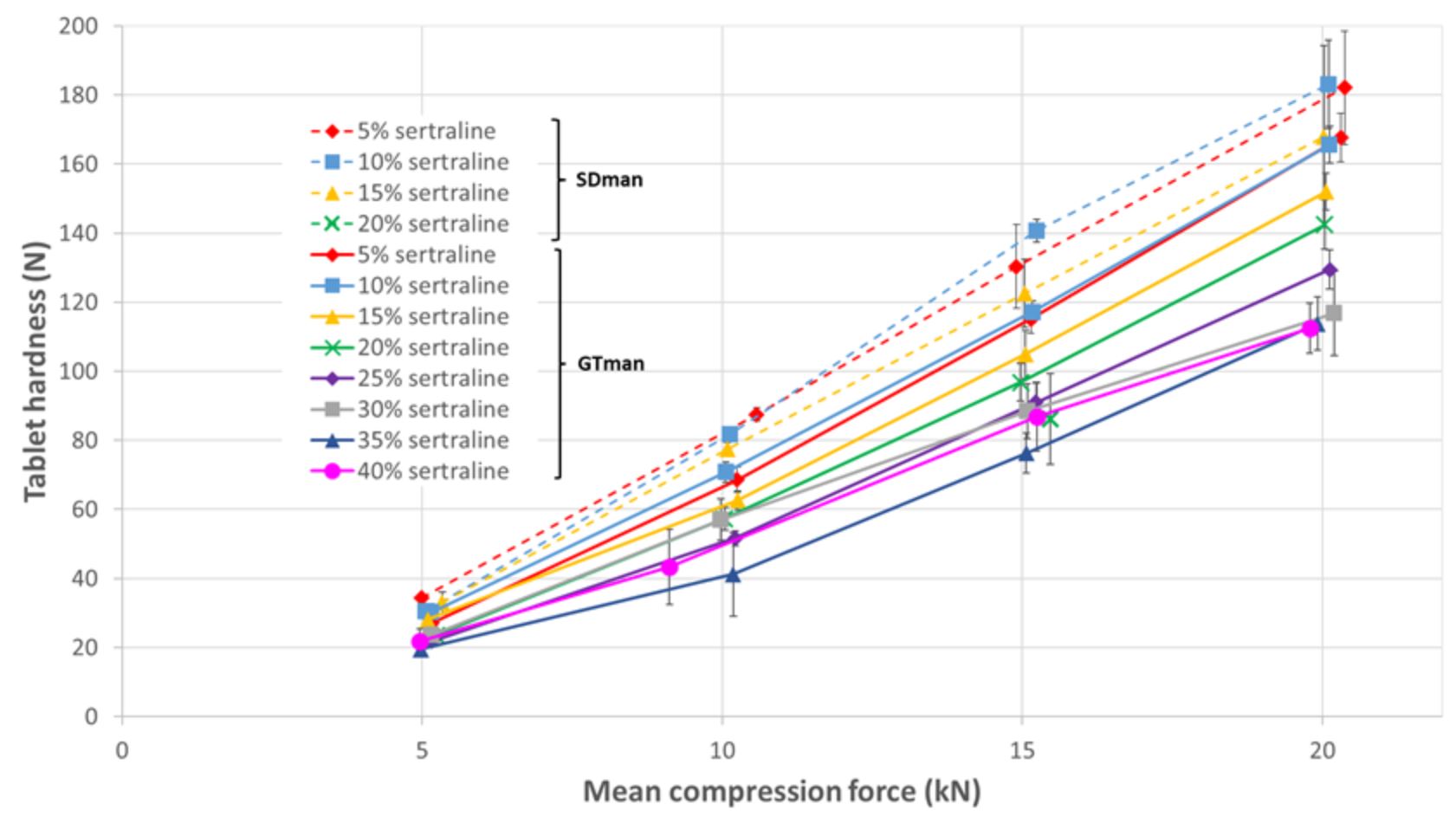
In the absence of precompression, SDman exhibited capping at every sertraline ratio. As the sertraline content increased, the compression force threshold for capping appearance decreased (capping at 20 kN compression force for 5% and 10% sertraline and 15 kN compression force for 15% sertraline (see figure 1). In these conditions, 100 N tablet hardness was accessible with 5 and 10% and not with 15% sertraline. With GTman, no capping was observed up to 25% sertraline ratio. At 30% sertraline ratio capping appeared at 20 kN compression force (high standard deviation of the tablet hardness). And with the decrease of tablet hardness due to the API ratio increase, a tablet hardness of 100 N was not reachable above 25% sertraline ratio.
Utilizing precompression, SDman began to show capping at 15% sertraline (with a high standard deviation of tablet hardness at 20 kN compression force), and tablets were not viable at 20% sertraline (as seen in figure 2). A tablet hardness of 123 N was achieved with 15% sertraline and a 15 kN compression force.
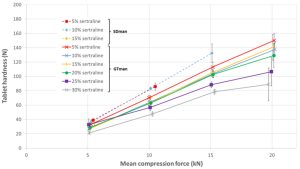
With GTman, acceptable tablets could be produced without capping up to 40 % sertraline content. Tablet hardness was observed to decrease with increasing sertraline ratio.
CONCLUSION
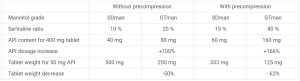
This study on tablet formulation with sertraline showed that using a DC mannitol with optimal tabletability offers the opportunity to increase API load or decrease tablet weight. Indeed, it was possible to load more API (+100 to 166%) when using PEARLITOL® 200 GT for the same tablet weight. Alternately, tablet weight could be reduced as well (-50% to -62%) while keeping the same API load (table 3).
Read the original article here
Source: Roquette, Philippe Lefèvre, Nicolas Descamps, Sébastien Croquet, Tessa Van Der Oost, Steve Amoussou-Guenou, Optimizing API Load and Minimizing Tablet Weight Leveraging (roquette.com)
See also our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


