Poly(D,l-lactide-co-glycolide) particles are metabolised by the gut microbiome and elevate short chain fatty acids
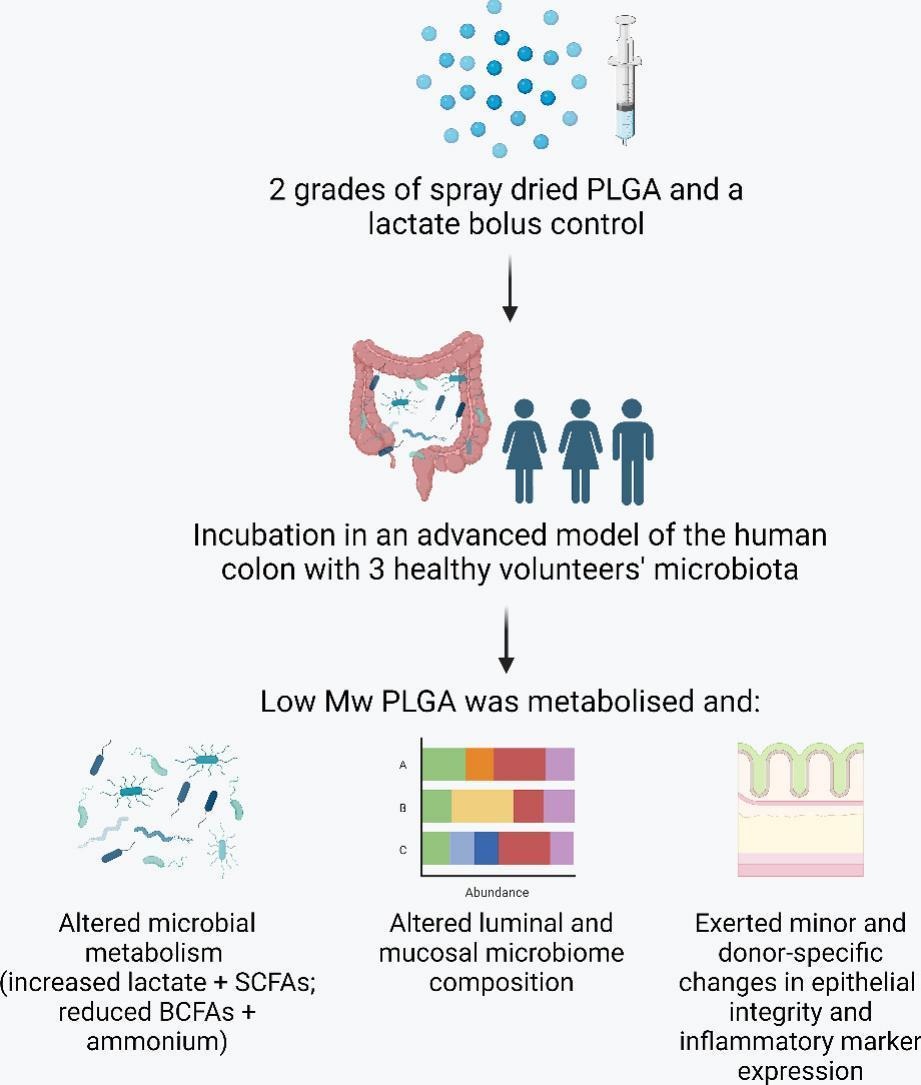
The production of short chain fatty acids (SCFAs) by the colonic microbiome has numerous benefits for human health, including maintenance of epithelial barrier function, suppression of colitis, and protection against carcinogenesis. Despite the therapeutic potential, there is currently no optimal approach for elevating the colonic microbiome’s synthesis of SCFAs. In this study, poly(D,l-lactide-co-glycolide) (PLGA) was investigated for this application, as it was hypothesised that the colonic microbiota would metabolise PLGA to its lactate monomers, which would promote the resident microbiota’s synthesis of SCFAs. Two grades of spray dried PLGA, alongside a lactate bolus control, were screened in an advanced model of the human colon, known as the M-SHIME® system. Whilst the high molecular weight (Mw) grade of PLGA was stable in the presence of the microbiota sourced from three healthy humans, the low Mw PLGA (PLGA 2) was found to be metabolised. This microbial degradation led to sustained release of lactate over 48 h and increased concentrations of the SCFAs propionate and butyrate. Further, microbial synthesis of harmful ammonium was significantly reduced compared to untreated controls. Interestingly, both types of PLGA were found to influence the composition of the luminal and mucosal microbiota in a donor-specific manner. An in vitro model of an inflamed colonic epithelium also showed the polymer to affect the expression of pro- and anti-inflammatory markers, such as interleukins 8 and 10. The findings of this study reveal PLGA’s sensitivity to enzymatic metabolism in the gut, which could be harnessed for therapeutic elevation of colonic SCFAs.
Highlights
- PLGA is susceptible to metabolism by the human gut microbiome.
- Digestion of PLGA with Mw 2000–2500 g/mol led to sustained lactate release.
- Lactate from PLGA was converted to propionate and butyrate in a colonic model.
- PLGA affected the abundance and composition of luminal and mucosal microbiota.
- PLGA exerted donor-specific effects on intestinal epithelial cells.
Introduction
The gut microbiome encompasses a hub of metabolic activity, whereby microbial and human metabolites interplay in a dynamic and bidirectional manner [1]. Small molecules produced by the intestinal microbiota have significant effects on host health. For example, indoles generated from bacterial tryptophan metabolism modulate the aryl hydrocarbon receptor within epithelial cells, safeguarding against inflammation and carcinogenesis [2]. Neuroactive compounds, such as serotonin and dopamine, are synthesised by bacteria in the GI tract and could play a role in human mood, motor control, sleep, and social behaviour [3].
SCFAs are an important group of microbial metabolites. Key SCFAs include acetate, propionate, and butyrate, and are produced through bacterial fermentation of dietary fibre in the colon. SCFAs activate cell-surface receptors such as free fatty acid receptor 3, succinate receptor 1, and hydroxycarboxylic acid receptor 2, leading to far-reaching effects on host physiology [1,4]. These effects include maintenance of epithelial barrier function, suppression of colitis, immunoprotection (e.g., against asthma), inhibition of carcinogenesis, cardiovascular protection, and improved glucose tolerance [1,[5], [6], [7], [8]].
Insight into the roles of gut microbial metabolites in human health has sparked interest in their development as potential medicines [4,9]. Among them, SCFAs have garnered substantial attention and investment, with the therapeutic potential of butyrate reported as early as the 1970s [10]. However, despite this early interest, no licensed pharmaceutical products currently exist for delivery of SCFAs, either in their original or pro-drug form [11]. Key reasons for this may lie in the molecules’ pungent odours and variability in clinical trials. An early clinical study reported that patients developed ‘socially unacceptable odour’ following injection with butyric acid, arising from the volatile compound’s presence in sweat and breath [12]. Secondly, clinical studies delivering SCFAs have produced varying results, with common pitfalls including small sample sizes, heterogenous endpoints, different administration routes, and biopharmaceutical challenges (i.e., achieving an effective concentration of drug in the colon) [[13], [14], [15]].
One notable success in SCFA delivery has been inulin-propionate ester (IPE), in which the SCFA propionate is covalently bound to the prebiotic inulin [16]. Upon arrival in the colon, the microbiota cleaves propionate from inulin and releases the SCFA at its target site [17]. Clinical studies have shown numerous benefits of IPE administration, including significant reductions in weight gain, intrahepatocellular lipid content, pro-inflammatory IL-8 in serum, and abdominal adipose tissue distribution in overweight and obese adults, alongside changes in faecal microbiome composition [[17], [18], [19]]. However, to exert these therapeutic benefits IPE must be administered in high dosages (10–20 g/day). Such dose sizes would increase the pharmaceutical burden on patients more than a typical oral dosage form providing <1.0 g of drug. An additional reason for the lack of translation of SCFAs as medicines could include their wide discussion in the literature, which may have impaired their patentability for pharmaceutical companies [20].
In light of these challenges, there is substantial opportunity for developing novel products that can increase colonic SCFA concentrations. The two most researched methods to increase colonic SCFA concentrations, without directly delivering SCFAs, include administration of prebiotics and probiotics [[21], [22], [23], [24]]. As with IPE, one crucial drawback of prebiotics is their dose; to elicit a therapeutic effect they generally need to be administered in large doses, which may place undue administration burden on patients [25]. Lactobacillus and Bifidobacteria represent the most widely researched genera of probiotics [26,27]. They are typically formulated as solid oral dosage forms with live cell counts from 108 to 1010 CFU/mL and have been clinically tested for a wide range of indications, namely irritable bowel syndrome, inflammatory bowel disease (IBD), antibiotic-associated diarrhoea, C. difficile infection, obesity/metabolic syndrome, neuropsychiatric conditions, and atopic dermatitis [[27], [28], [29], [30], [31]]. Many strains within these genera can directly produce SCFAs or lactate as a precursor for microbial SCFA synthesis in the intestines (Fig. 1) [21,32]. Whilst probiotics have received promising results in a myriad of studies, they face multiple pharmaceutical and biopharmaceutical challenges. For instance, formulation processes can frequently reduce the number of live bacteria within products due to heat, mechanical stress, oxygen, and excipient exposure [33]. Further, gastric acid, enzymes, and bile salts can inactivate probiotics in vivo, reducing the number of live bacteria that reach the colon [34]. Even probiotics that reach the colon alive can have insufficient effects due to lack of mucosal colonisation [35]. Hence, there is a requirement for alternatives to prebiotics and probiotics for augmentation of colonic SCFAs.
Continue reading the full research paper as PDF: PLGA particles are metabolised by the gut microbiome and elevate short chain fatty acid
or continue reading here
Laura E. McCoubrey, Fabiana Ferraro, Nidhi Seegobin, Jérémy Verin, Haya A. Alfassam, Atheer Awad, Massimo Marzorati, Lynn Verstrepen, Jonas Ghyselinck, Julie De Munck, Jelle De Medts, Evi Steppe, Valerie De Vleeschhauwer, Gilles De Rocker, Alexandra Droesbeke, Melanie De Rijck, Sara Vanthoor, Frédéric Moens, Juergen Siepmann, Florence Siepmann, Simon Gaisford, Mine Orlu, Abdul W. Basit,
Poly(D,l-lactide-co-glycolide) particles are metabolised by the gut microbiome and elevate short chain fatty acids,
Journal of Controlled Release, Volume 369, 2024, Pages 163-178,
ISSN 0168-3659,
https://doi.org/10.1016/j.jconrel.2024.03.039.

