Quality by Design applied to the development of soft gelatin capsules
The pandemic we are experiencing made us realize the impact and need for medication in patients’ lives. These, in turn, are substances that contain drugs in their composition, the so-called active principles responsible for alleviating various symptoms. In this sense, the article will address some fundamental aspects of quality by design applied to the development of soft capsules, advocating that we think of the patient and quality not as a finish line but as a starting point throughout the product’s life cycle, which certainly drives innovation and adaptability to change, after all, change is the only constant! It is worth remembering that the pharmaceutical industry is undoubtedly one of those that invest the most in Research & Development globally, increasingly driving the world through innovation, technology and opening the doors to new horizons.

The pharmacotechnical development of drugs involves factors inherent to production technology and quality control, ensuring safety and therapeutic efficacy. However, the clinical efficacy of a drug is not attributed solely to its intrinsic pharmacological activity, so the evaluation of aspects related to the physicochemical and pharmacokinetic properties of the active pharmaceutical ingredients, to the excipients used in the formulation, and to the manufacturing process and technology is considered indispensable to achieve success and ensure bioavailability in vivo.
It is essential to clarify that quality in the pharmaceutical industry can only be considered adequate if it works preventively; that is, it must prevent failures from occurring. For this reason, the only way to do this is through risk management. This can be characterized as a process of selecting measures to prevent and control problems based on a risk assessment, aiming to improve decision-making, reduce subjectivity, establish well-established methods, and above all, provide confidence to regulatory bodies.
On the other hand, instituting a process like this will require time, trust, and communication between the areas, and in my opinion, the change in the company’s mindset will be the most impacted. Therefore, the biggest challenge is to make those involved understand tangibly the value that risk analysis has. When carried out efficiently, mapping the processes, evaluating the risks involved in each step, defining their effects, and the risk mitigation or control actions, this tool is undoubted of great value to the company[1].
It is worth pointing out that the work is hard, and the return is not immediate; however, in the medium and long term, the implementation of the so-called quality culture generates effective and high impact returns, such as reducing rework, the mitigation of failures in processes and products, increased productivity and, significantly, increased credibility of companies before their primary customers, the main one, the patient.
Moreover, it enables error reduction, improving manufacturing efficiency and generating savings for the industry, as well as improving product quality, avoiding in many cases even the recall of products, in addition to decisions based on science-based on risk, with the promotion of a multidisciplinary approach in the sectors involved, since it can enhance the encouragement of a preventive approach in all processes, managing to add value to the product, preventing the application of restrictive and unnecessary measures. To achieve this quality goal reliably, there must be a comprehensive and adequately implemented pharmaceutical quality system incorporating risk management[2].
Regarding pharmaceutical products, although there are various stakeholders, including pharmacists, physicians, and nurses, patient protection must be considered of paramount importance. Therefore, quality must be an essential requirement in healthcare facilities, where risk management must come from the top down. The leadership needs to believe in its importance, commit to replicating this culture throughout the institution, and have everyone involved follow the letter’s processes.
Industry Perspective & Implementation of QbD
From the pharmaceutical industry’s perspective, QbD requires the development of a fundamental scientific understanding of critical processes and product attributes, the establishment of design controls and testing based on product quality and within the limits of scientific understanding and use of knowledge gained over a product’s life cycle to operate in an environment of continuous improvement.
Implementing the concept of Quality by Design (QbD) proposes a systematic approach to development, starting with predefined objectives emphasizing product understanding and process control. Based on the scientific approach and the management of quality risk, focusing on the product life cycle, through which it passes, from the beginning of development, commercialization until its discontinuation, having several customers (stakeholders)—each one with specific needs and expectations, causing a practical impact on the company’s strategic planning. Allowing organizing ideas and making decisions with more assertiveness and creativity, directing innovation and product centered on the patient, generating value, and providing short, medium, and long-term solutions for the business[3].
Putting the user at the center of the process makes it easy to realize that the value of innovation can sometimes be in the details because this concept looks for evidence in places where not everyone looks. Many companies overestimate technological innovation, buying new machines, and over-modernizing industrial parks, but forget that innovation is also about people. Thus, this concept will help managers understand that innovation is, above all, what impacts patients’ lives, following the company’s pillar, valuing human beings, and respecting their differences.

The application of the concept and consolidation of the team, the realization of the pharmaceutical product development report, and qualification of the process performance. In addition, to provide a scale-up for production in a more powerful way and its continuous verification (from registration to product launch), and parallel to these activities, training will be given on this subject to establish the new culture in the company, since focusing on customer needs and their natural desires, which are in constant transformation, demonstrates that it is possible to dare and innovate, quickly and well done.
The pharmaceutical sector is in constant evolution. The harmonization of production standards to guarantee the efficacy, safety, and quality of medicines, is one of the biggest challenges. In this approach, quality is inversely proportional to variability, and the implementation of the QbD concept is a promising tool for the pharmaceutical industry, as it allows the production of drugs through risk prediction, enabling cost reduction. The implementation of this concept requires new technologies and technologies, a change in the quality concept of the company[4].
Quality by Design Definition
Quality by Design (QbD) provides a scientific, risk-based, holistic, proactive approach to enhanced product development/understanding (product formulation, process, and device) and ensures consistent desired product quality, including performance over the product life cycle including continual improvement.
- Quality Target Product Profile (QTPP) describes the design criteria for the product and should therefore form the basis for developing the CQAs, CPPs, and control strategy.
- Critical Quality Attributes (CQA): A physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality.
- Critical Process Parameter (CPP): A process parameter whose variability impacts a CQA should be monitored or controlled to ensure the process produces the desired quality.
- Critical Material Attribute (CMA): A physical, chemical, biological, or microbiological property or characteristic of an input material within an appropriate limit, range, or distribution to ensure the desired quality of output material.
Quality by Design Principles
Target Quality: a shared objective on project outcomes and product specifications.
Design: QbD already starts in the early phase and intensifies throughout the project scale-up. Core principles:
- Must be acknowledged by Top Management as a way of conducting projects.
- QbD is performed as teamwork, so diverse groups, including the client, breaking silos, process experts, and fresh eyes.
- QbD is an investment at the startup, generating valuable knowledge on the product and reducing risks and global timelines.
Overview: Regulatory expectation
It is essential to highlight that after the publication of the RDC 301/2019, which presents the Good Manufacturing Practices (GMP) for pharmaceutical products, the risk analysis has received more attention from professionals in this area, is considered an essential requirement for the evaluation of the drug manufacturing process. In the international scope, the ICH Q9 guide brought in a more tangible way the implementation of risk management in the pharmaceutical industry, becoming more scientific, being an essential component in the maintenance of quality throughout the life cycle of products, allowing the research and evaluation of risk in all stages of manufacturing activities.
Thus, we must think of the patient and quality as a continuous attitude, not an episodic event! Because of the above, I am fully convinced that the change must be cultural, where the focus must be on quality to ensure this paradigm shift. Reproducible manufacture medicines that are safe and effective.
The US Food and Drug Administration (FDA) encourages risk-based approaches and the adoption of QbD principles in drug product development, manufacturing, and regulation. FDA’s emphasis on QbD began with the recognition that increased testing does not necessarily improve product quality, and quality must be built into the product.
The concept and definition of Quality Target Product Profile (QTPP), Critical Quality Attribute (CQA), Critical Process Parameters (CPPs), Design Space and Control strategy are described in:
- ICH Q8 Pharmaceutical Development [5].
- ICH Q9 Quality Risk Management [6].
- ICH Q10 Pharmaceutical Quality System[7].
- FDA Guidance for Industry: Process validation.
- EMA Guideline on process validation.
- RDC Nº 301/2019 – ANVISA.
The steps of Quality by Design
It begins with the “target product profile (TPP),” which describes the use, safety, and efficacy of the product [8].
“Quality target product profile,” which describes the quantitative information on clinical safety and efficacy during the product development stage, is defined and used by the formulation and process personnel.
Knowledge of the active ingredient, potential inactive substances, and process operations are collected into a knowledge space. If further research is needed, risk assessment is done to predict the gaps in the knowledge.
Formulations and “Critical material (quality) attributes” of the finished product, which should be controlled, are defined to meet the target quality product profile.
Following the manufacturing process of the product of which the critical material attributes are determined, A Design Space or other representation of process understanding is established by combining experiences with prior knowledge to utilize other tools.
A “control strategy” is created for the entire process, including input material controls, process controls, monitoring, design spaces around single or multiple unit operations, and/or finished product test. The control strategy should include the expected changes in scale, and risk assessment can guide the control strategy.
Following this, monitoring is carried out, and the process is updated to guarantee the sustainability of quality.
Outcome of QbD
The risk of delay in registration and launch can also impact the financial success of a marketed product. Research shows that a product that is six months late to market loses one-third of the potential profit over the product life cycle and that implementing the QbD concept can decrease development costs by up to 50 percent and production costs by 9 percent, enabling profits to increase by 22 percent [9].
Any reduction in the total drug discovery period in the market should improve the profitability of the company. In this regard, pharmaceutical companies are focusing on strategies to optimize the product life cycle as it allows them to maximize the initial growth of the product on the market, sustain peak sales while the product is patented, and delay the decline post-patent expiration for as long as possible. This should enhance the return on investment over a product’s life cycle, allowing the company to recover development costs and make further investments in R&D.
By putting QbD into practice, it will become disseminated as a strategic resource capable of positioning competitive advantages compared to the market, i.e., a customer-centric thinking model that seeks innovative and meaningful solutions. With a patient-oriented approach, this concept is structured by the Empathy, Collaboration, Research, and Experimentation pillars.
The practice lies in developing products and making them and the strategic processes adopted throughout the product life cycle. This will enable the company to innovate in different sectors and market areas, aiming to develop ideas that improve the function, value, and appearance of products for the mutual benefit of the customer and the Procaps Group.
Advantages to QbD Application

Key advantages to QbD application include [10]:
- Decreases stability problems.
- Increase process assertiveness.
- Increase traceability.
- Minimize the number of reprocesses.
- Increases efficiency in the bench stage.
- Lower cost in development.
- Decreases the problems faced in the scale-up stage.
- Achievement of project goals.
- Need for regulatory adequacy (GMP).
- Maximization of return on investment.
- Greater control of project spending.
- Increases the safety and efficacy of the drug.
- A systematic approach to development.
- Better process knowledge.
- Fewer review cycles, Faster review approval.
- Pharmaceutical industry viability and culture change.
Challenges to QbD Application
Critical challenges to QbD Application include:
- Identify genuine risks based on a scientific approach.
- Open application of QbD for the execution of the action approach.
- Finding the right balance between classic approach and QbD concerning commercial viability,
- Valuable knowledge generated, and how to share it and keep it alive.
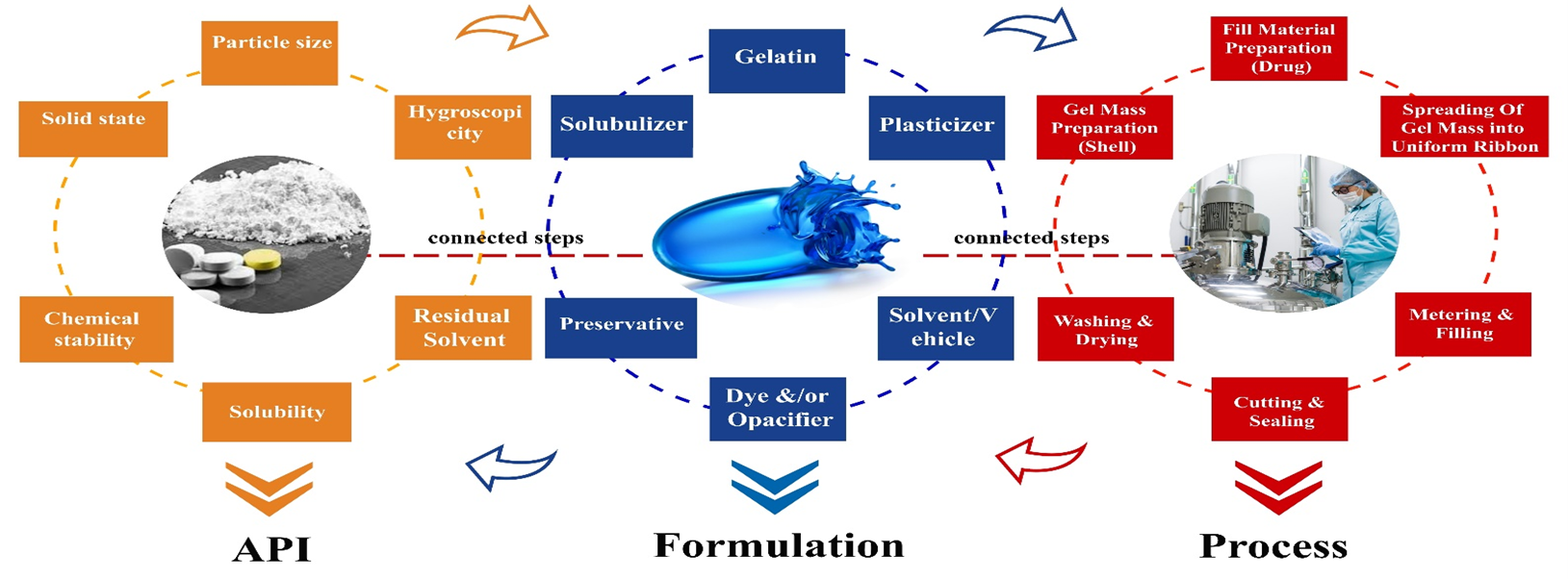
Softgels
Soft gelatin capsules (soft or elastic soft capsules/softgels) consist of hermetically sealed soft shells. Soft gelatin capsules are prepared by adding a plasticizer, such as glycerin or polyhydric alcohol (for example, sorbitol), to the gelatin, and the plasticizer makes the gelatin elastic. Soft gelatin capsules come in various shapes, such as spherical, elliptical, oblong, and special tubes with and without twists. They can contain non-aqueous liquids, suspensions, or pasty materials. They are essential for containing volatile drug substances or susceptible to degradation in the presence of air [11,12].
These, in turn, are formed by the rotary die process. Depending on the polymer that forms the shell, they can be subdivided into soft gelatin or non-gelatin capsules. Most soft capsules are made of gelatin due to the unique physical properties that make them an ideal excipient for the rotary die process. Soft capsules based on non-gelatin alternatives are plant-derived and/or synthetic [13].
The shell of a softgel capsule consists of gelatin, a plasticizer, or a combination of plasticizers and water. In addition, it may contain preservatives, colorants, opacifiers, flavorings, and sweeteners, possibly sugar to impart chewable characteristics, gastro-resistant substances, and, in exceptional cases, even active compounds. Water serves as a solvent to form a melted gelatin mass with fluid viscosity at 60-70 °C. The ratio by weight of water to dry gelatin can vary from 0.7 to 1.3, depending on the viscosity of the gelatin being used. After capsule formation, most water is removed by drying, leading to capsules with a residual water content of around 4-10% [13].
Gelatin capsules consist of a liquid or a semi-solid matrix inside a one-piece gelatin outer layer. The drug compound itself may be in solution or suspension in the capsule filling matrix. The characteristics of the filling matrix may be hydrophilic (e.g., polyethylene glycol), lipophilic (e.g., triglyceride vegetable oils), or a combination of hydrophilic and lipophilic ingredients [14]. Ingredients that are solid at room temperature can also be encapsulated in soft capsules, provided they are at least semi-solid below about 40 °C.
In turn, this type of pharmaceutical form has several advantages, among them: ease of swallowing; precise liquid filling unit volume offers greater precision and batch-to-batch capsule consistency; consistent manufacturing requirements: more precise liquid filling composition, mixing and distribution facilitate manufacturing; liquid mixtures are more homogeneous; increased bioavailability: absorption and bioavailability can be enhanced by formulating compounds in solution, including solubilizes and absorption enhancers if needed; water-insoluble drugs can be formulated in soft capsule; increased stability and safety: airtight seal protects from air and cross-contamination; gelatin shell can be formulated to block ultraviolet light; flexibility allows custom shapes and sizes suitable for oral, topical, chewable and suppository administration; transport: encapsulated liquid dosage formulations become highly versatile for consumers/patients [12].
Manufacturing is a simple process with fewer steps than other solid oral technologies, making it straightforward for QbD applications. The QbD principles are readily applicable to soft capsules product concepts, starting with the project and iterating all along the project life cycle. Projects can be received at any stage of development and progress with a risk-based approach.
Emphasis will be given to the different Critical Quality Attributes (CQAs) that should be evaluated as part of the QbD (Quality by Design) strategy during the development of drug products containing gelatin.
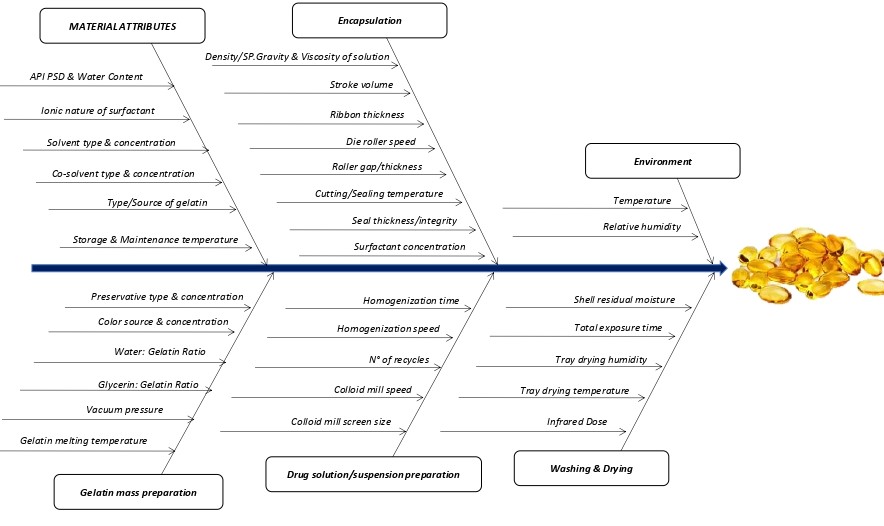
Conclusion
Therefore, we conclude that the pharmaceutical development based on the product life cycle approach should be performed under the concepts and principles previously mentioned, incorporating the Good Manufacturing Practices (GMP), meeting the expectations of regulatory agencies. In this way, quality should be controlled in each step of the process to maximize the probability of the product reaching the patient achieving its quality attributes and specifications, considering all those involved: multidisciplinary team, suppliers, and final customer.
Quality by Design is intended to enhance process knowledge and is based on existing guidance and reference documents. It can be viewed as a process defined by document requirements as per process knowledge and understanding. It can be applied to legacy and new products, but the supporting document package may differ. The QbD suite of documents is “alive.” They can and should be revised as the knowledge base changes. Implementing pharmaceutical QbD aims to reduce product variability and defects, thereby enhancing product development and manufacturing efficiencies and post-approval change management.
Finally, QbD is a challenge in implementation from an industry perspective because it has yet to fully embrace its application to pharmaceutical product development. Given the need for mapping and controlling the risks observed during the product’s life cycle, however, applying this concept, there will undoubtedly be a significant improvement in the quality and effectiveness of the medicine regarding patient treatment.
 Download the full article as a PDF here
Download the full article as a PDF here
Article information: Antraco, V.J.; Quality by Design applied to the development of soft gelatin capsules. PharmaExcipients 2021.
Source

Vitor Jacó Antraco is a senior pharmaceutical development analyst at Softigel by Procaps Group. Tel: +55113405-5858. He holds a master’s degree in metabolic/molecular biochemistry from the Federal University of São Paulo (UNIFESP) and a Master of Business Administration from the Getúlio Vargas Foundation (FGV) in Health Management a bachelor’s degree in pharmacy/biochemistry from the Mackenzie Presbyterian University. He has worked in Oncology / Hospital Hematology, Quality Assurance/ Validation, Galenic Development and Pharmacotechnical Development. -R& D.
References
- Junker, B.H. Building a Business Case for Biopharmaceutical QbD Implementation (Peer Reviewed). 2012.
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783, doi:10.1208/s12248-014-9598-3.
- Calcott, P.H. How QbD, and the FDA Process Validation Guidance Affect Product Development and Operations, Part. Bioprocess Int. 2011, 9, 12–17.
- Sangshetti, J.N.; Deshpande, M.; Zaheer, Z.; Shinde, D.B.; Arote, R. Quality by design approach: Regulatory need. Arab. J. Chem. 2017, 10, S3412–S3425.
- Guideline, I.C.H.H.T. Pharmaceutical development. Q8 (2R). As for Revis. August 2009.
- Guideline, I.C.H.H.T. Quality risk management. Q9, Curr. step 2005, 4, 408.
- (ICH), I.C. on H. ICH Harmonised Tripartite Guideline: Pharmaceutical Quality System Q10 (Current Step 4 version). 2008.
- Mesut, B.; Ȍzsoy, Y.; Aksu, B. The place of drug product critical quality parameters in quality by design (QbD). Turk J Pharm Sci 2015, 12, 75–92.
- Fuhr, T.; Holcomb, M.; Rutten, P. Why quality-by-design should be on the executive team’s agenda. Dev. New Strategy. New Times. Mckinsey Co. 2009, 195–203.
- Nadpara, N.P.; Thumar, R. V; Kalola, V.N.; Patel, P.B. Quality by design (QBD): A complete review. Int J Pharm Sci Rev Res 2012, 17, 20–28.
- Gullapalli, R.P. Soft gelatin capsules (softgels). J. Pharm. Sci. 2010, 99, 4107–4148.
- Augsburger, L.L.; Hoag, S.W. Pharmaceutical dosage forms: capsules; CRC Press, 2017; ISBN 1841849774.
- Reich, G. Formulation and physical properties of soft capsules. Pharm. Capsul. Pharm. Press. London 2004, 201–212.
- Aulton, M.E.; Taylor, K. Aulton’s pharmaceutics: the design and manufacture of medicines; Elsevier Health Sciences, 2013; ISBN 0702042900.

