Raw Material Variability in Biomanufacturing: A Case Study Using Poloxamer 188
Introduction & Aim
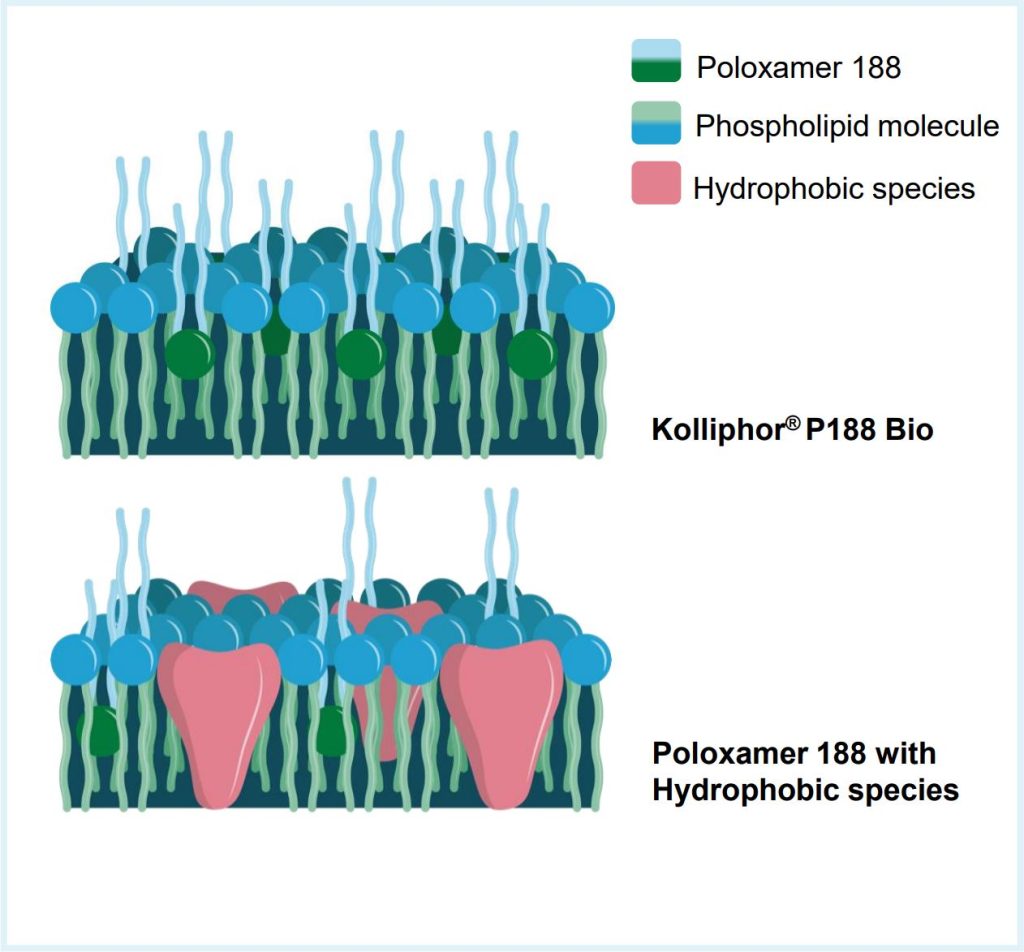
Media for cell culture has evolved from recipes containing plant or animal-derived products, to fully chemically defined (CD) formulations [1] as an effort to reduce variability in bioprocess performance due to raw materials. A critical ingredient, poloxamer 188, has a history of variability [2] due to hydrophobic species (Fig. 1).
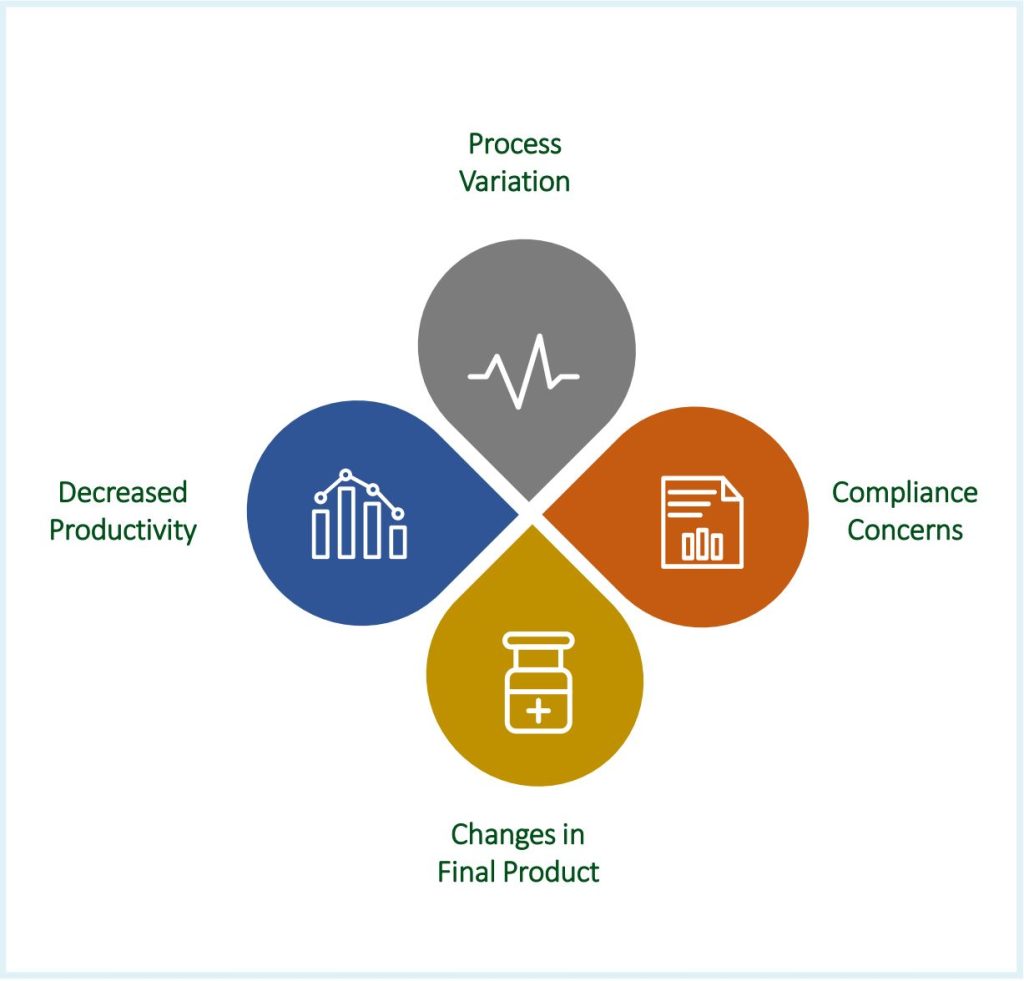
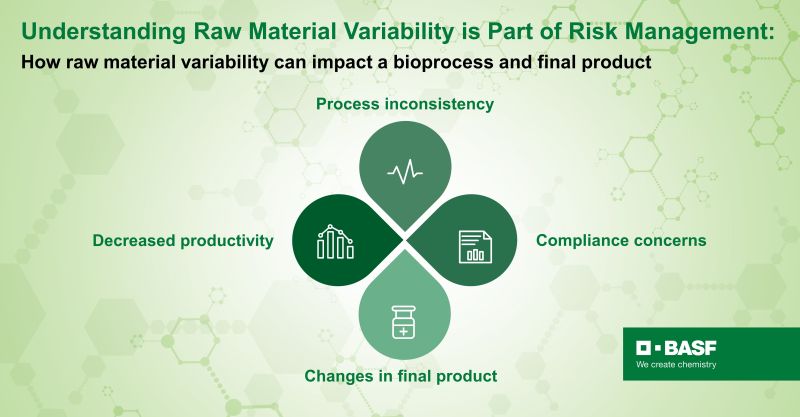
Intra-lot, lot-to-lot and vendor-to-vendor variability in raw materials can bring forth an imbalance of some key media ingredients which can directly impact the bioprocess by adding variations, losses in yield and adding concerns for compliance and changes in final product (Fig. 2). The present work looks to showcase the consistency in cell performance of different lots of critical raw material, Kolliphor® P188 Bio, a GMP version of poloxamer 188 that is a fit-for-function shear protectant used in biomanufacturing. In our study, we compared cell performance within, Intra-Lot, and between, Inter-Lot, two lots of Kolliphor® P188 Bio.
Materials & Methods
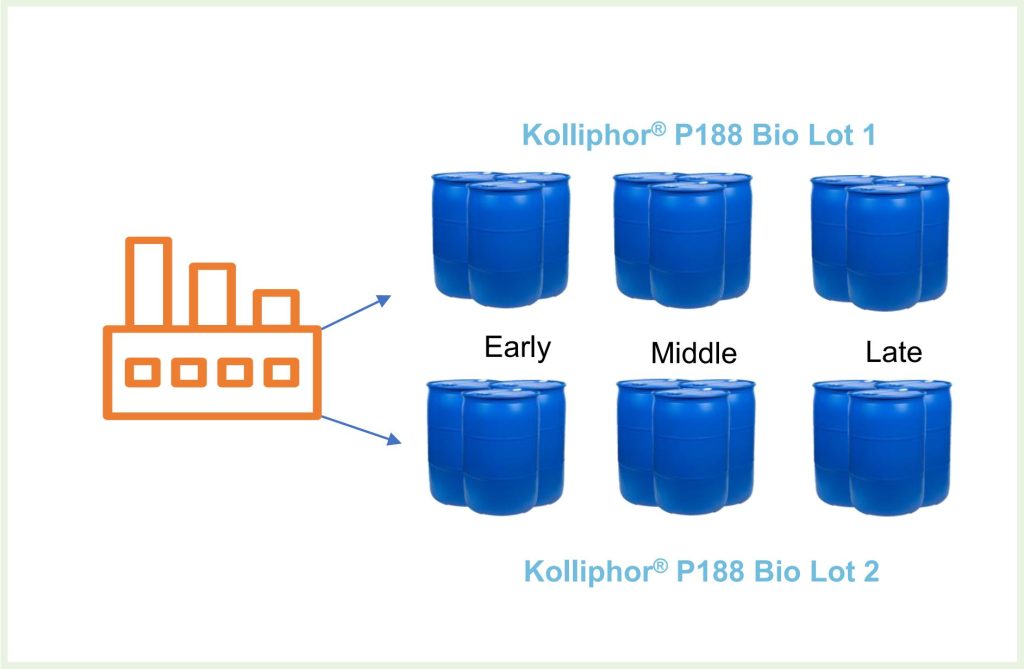
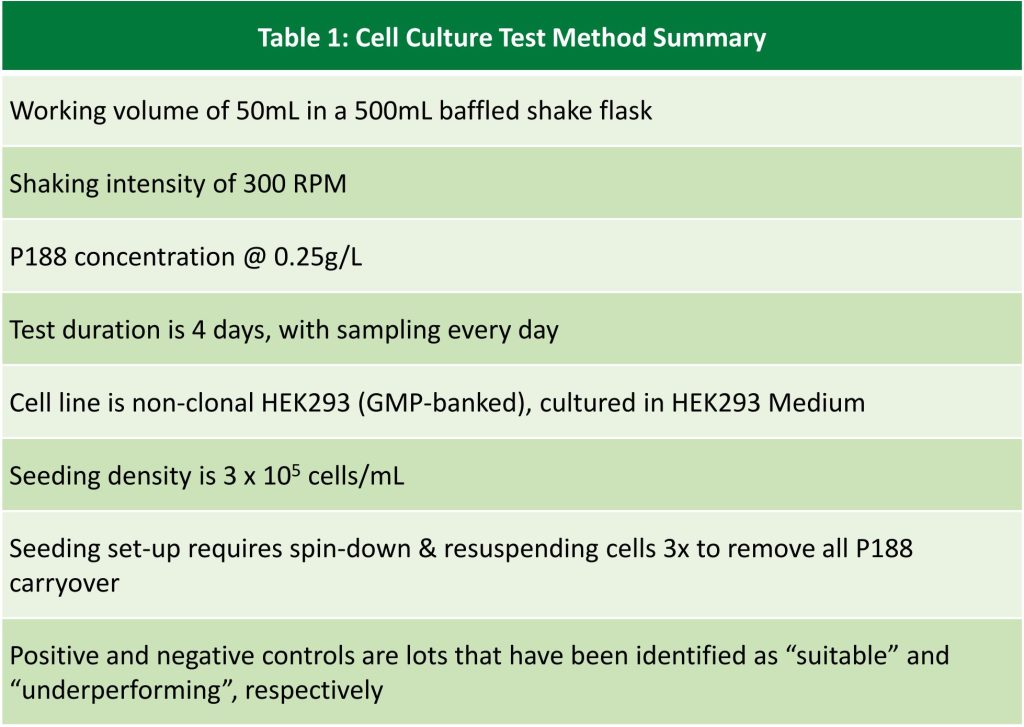
Drums of two lots of Kolliphor® P188 Bio were allocated into one of these categories: Early, Middle or Late depending on the time they were filled during the manufacturing cycle (Fig. 3). From these categories, we sampled n>>1 drums and used HEK293 cells to test cell viability over a 4-day period (Table 1). Samples were tested in triplicate.
Cell performance was analyzed using analysis of variance (ANOVA), where we compared cell performance in the presence of different drums from two lots of Kolliphor® P188 Bio. When comparing lots from within a lot, Intra-Lot, we found that both Kolliphor® P188 Bio lots had good performance indicated by high cell viability and there was no significant statistical difference in cell performance between the different groups of drums within a lot. When comparing drum groups of two lots of Kolliphor® P188 Bio, Inter-Lot, we found no statistically significant difference in cell performance between drum groups between the two lots. We compared these results to the cell performance of a Non-Bio Lot of poloxamer 188 which had much lower cell performance than the Kolliphor® P188 Bio lots tested.
See the full brochure on “Raw Material Variability in Biomanufacturing” here
(click the picture to download the brochure)
Source: BASF brochure “Raw Material Variability in Biomanufacturing”
Do you need more information or a sample of excipients by BASF?