A new insight into the mechanism of the tabletability flip phenomenon
Abstract
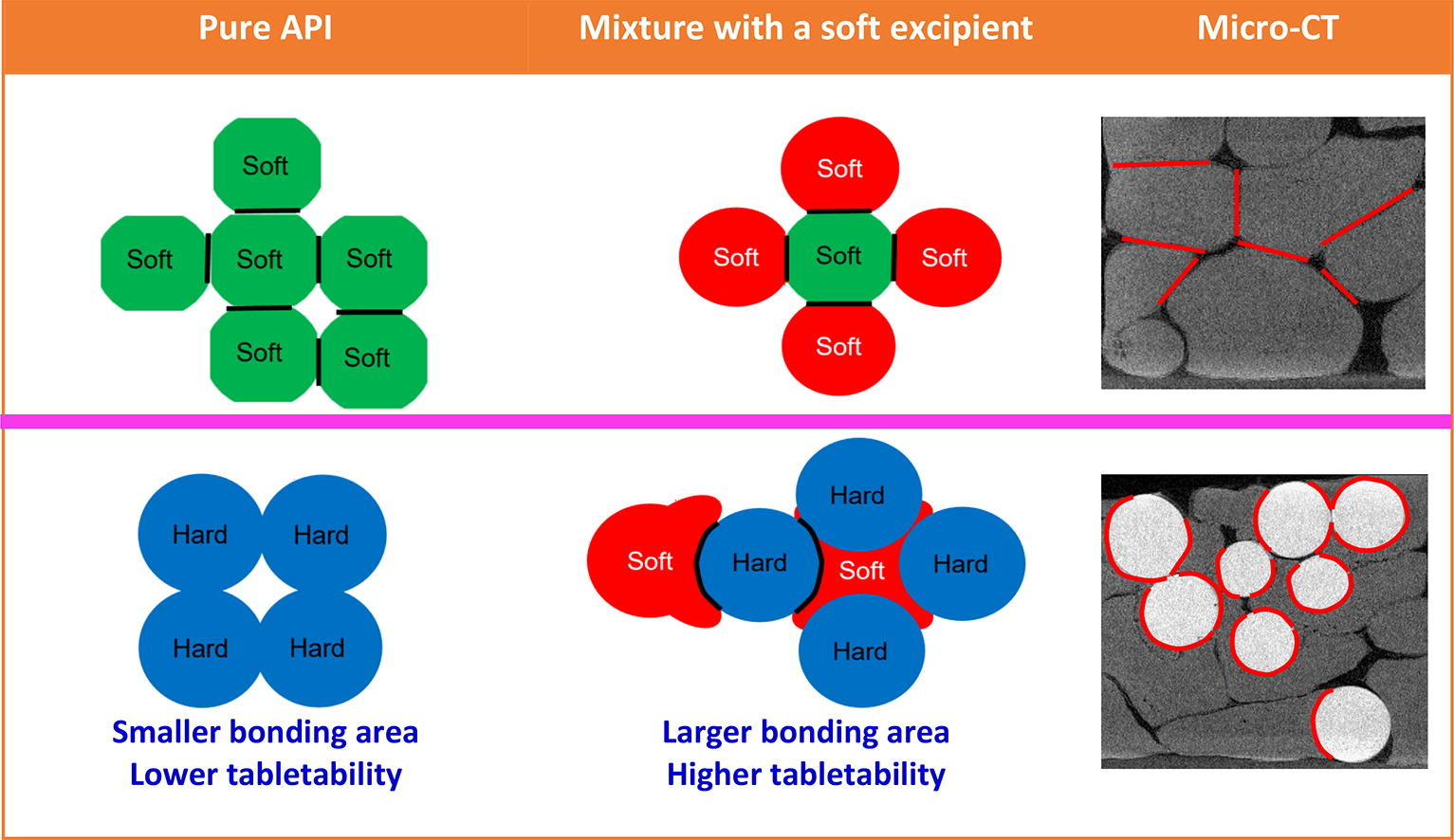
Tabletability is an outcome of interparticulate bonding area (BA) – bonding strength (BS) interplay, influenced by the mechanical properties, size and shape, surface energetics of the constituent particles, and compaction pressure. Typically, a more plastic active pharmaceutical ingredient (API) exhibits a better tabletability than less plastic APIs due to the formation of a larger BA during tablet compression. Thus, solid forms of an API with greater plasticity are traditionally preferred if other critical pharmaceutical properties are comparable. However, the tabletability flip phenomenon (TFP) suggests that a solid form of an API with poorer tabletability may exhibit better tabletability when formulated with excipients. In this study, we propose another possible mechanism of TFP, wherein softer excipient particles conform to the shape of harder API particles during compaction, leading to a larger BA under certain pressures and, hence, better tabletability. In this scenario, the BA-BS interplay is dominated by BA. Accordingly, TFP should tend to occur when API solid forms are formulated with a soft excipient. We tested this hypothesis by visualizing the deformation of particles in a model compressed tablet by nondestructive micro-computed tomography and by optical microscopy when the particles were separated from the tablet. The results confirmed that soft particles wrapped around hard particles at their interfaces, while an approximately flat contact was formed between two adjacent soft particles. In addition to the direct visual evidence, the BA-dominating mechanism was also supported by the observation that TFP occurred in the p-aminobenzoic acid polymorph system only when mixed with a soft excipient.
Introduction
The oral tablet is a preferred pharmaceutical dosage form for drug delivery due to its excellent physical and chemical stability, ease of administration, precise dosing, customized dissolution performance, high manufacturing efficiency and low manufacturing cost (Nyol and Gupta, 2013, Rudnic and Edward, 2002, Ubhe and Gedam, 2020). For a tablet product to be successful, it must possess adequate mechanical strength to remain intact post manufacturing until administration by consumers. This necessitates sufficient tabletability – the ability of a powder to form a tablet of specific strength under the effect of compaction pressure (Sun and Grant, 2001).
Tabletability is determined by the interplay between bonding area (BA) and bonding strength (BS) between the particles, with the former influenced by their mechanical properties, particulate properties, and compaction conditions, and the latter influenced by the nature of the materials (Sun, 2011). For examples, plastic deformation of ductile powders during die compression also explains the strength anisotropy (stronger along the radial direction than the compaction direction) (Galen and Zavaliangos, 2005). Successful simulation of plastically deforming powder compaction requires accurate mechanical properties as input parameters (Cocks and Sinka, 2007). Particle size can affect, sometimes significantly, tabletability of materials (Mckenna and Mccafferty, 1982, Paul et al., 2019). It is also well known that tableting performance is also affected by the process parameters employed during tablet manufacturing (Sinka et al., 2009). Typically, a more plastic active pharmaceutical ingredient (API) can undergo more extensive plastic deformation under compression, resulting in a larger BA and better tabletability (Chang and Sun, 2017, Chen et al., 2022, Hu et al., 2019). Therefore, a more plastic solid form of an API is generally preferred for formulation development due to its enhanced tabletability. However, the occurrence of the tabletability flip phenomenon (TFP), where a less plastic API with poorer tabletability can exhibit better tabletability when formulated in the same excipient matrix (Paul et al., 2020, Wang et al., 2023), calls for caution in such preferences. Previous studies have indicated that TFP tends to manifest when the compaction pressure is high or when there is a significant difference in plasticity between two solid forms (Wang et al., 2023). Understanding the mechanism of TFP is critical for predicting the compaction properties of mixtures, providing invaluable insights for efficient and reliable tablet formulation design.
A previously proposed mechanism suggests that TFP results from a shift in the dominating factor in the BA-BS interplay from BA to BS upon formulation (Paul et al., 2020). In other words, the previous mechanism assumes that BAs of mixtures do not differ significantly when two APIs are mixed with a more plastic excipient. Consequently, the tabletability of a mixture is higher for the less plastic API, which exhibits a higher BS. This mechanism holds true only if the excipient is significantly softer than both API forms, satisfying the assumption of comparable BA in the tablets of the two mixtures. However, this condition was not always satisfied in the previous cases of TFP. Hence, a different mechanism is required to explain the TFP when the plasticity of the excipient is not significantly higher than both API solid forms. A correct mechanistic understanding of the TFP is essential for developing effective strategies to either overcome the TFP or guide the design of tablet formulation for better tabletability (Sun, 2009). Accordingly, we carried out this work to explore an alternative mechanism of TFP.
Read more here
Materials
PlayDoh (Hasbro, Pawtucket, RI, USA), glass beads with a diameter of 1 mm (Thermo Fisher Scientific, Fair Lawn, NJ, USA), magnesium stearate (MgSt; Covidien; Dublin, Ireland), p-Aminobenzoic acid α form (ABAα, Thermo Scientific Chemicals, Waltham, MA, USA), Microcrystalline cellulose (MCC; Avicel PH105, FMC; Newark, DE, USA), Calcium Hydrogen Phosphate Dihydrate (DCPD, Emcompress®, JRS Pharma; Patterson, NY, USA), and Methanol (Sigma-Aldrich; St. Louis, MO, USA).
Zijian Wang, Chenguang Wang, Yiwang Guo, Deepak Bahl, Alex Fok, Changquan Calvin Sun, A new insight into the mechanism of the tabletability flip phenomenon, International Journal of Pharmaceutics, 2024, 123956, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123956.
Read also our introduction article on Orally Disintegrating Tablets (ODTs) here:


