Formulation and Delivery Technologies for mRNA Vaccines
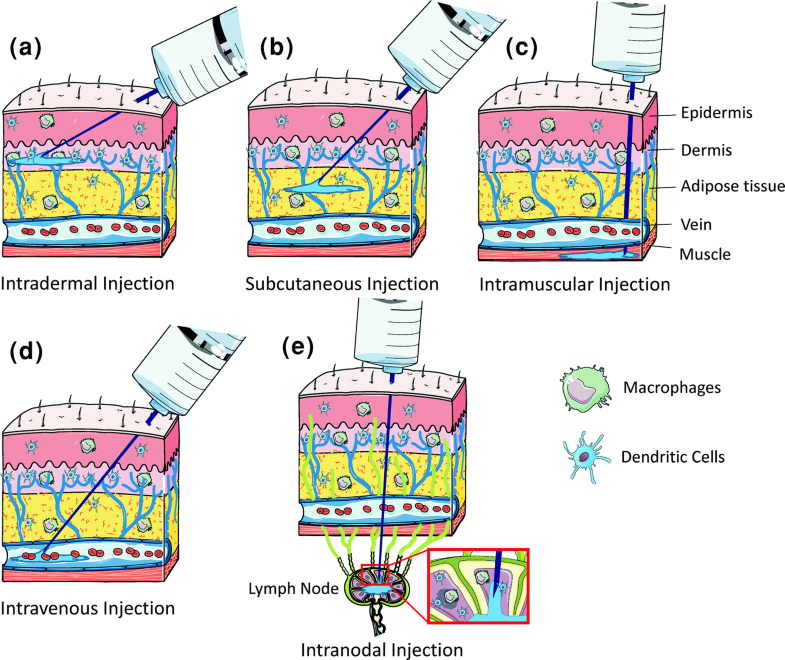
mRNA vaccines have become a versatile technology for the prevention of infectious diseases and the treatment of cancers. In the vaccination process, mRNA formulation and delivery strategies facilitate effective expression and presentation of antigens, and immune stimulation. mRNA vaccines have been delivered in various formats: encapsulation by delivery carriers, such as lipid nanoparticles, polymers, peptides, free mRNA in solution, and ex vivo through dendritic cells. Appropriate delivery materials and formulation methods often boost the vaccine efficacy which is also influenced by the selection of a proper administration route. Co-delivery of multiple mRNAs enables synergistic effects and further enhances immunity in some cases. In this chapter, we overview the recent progress and existing challenges in the formulation and delivery technologies of mRNA vaccines with perspectives for future development.
Introduction
Since the first use of in vitro transcribed messenger RNA (mRNA) to express an exogenous protein in mice in 1990 (Wolff et al. 1990), mRNA has evolved into a versatile platform spanning many therapeutic and prophylactic fields (Hajj and Whitehead 2017; Xiong et al. 2018; Li et al. 2019; Patel et al. 2019b; Pardi et al. 2020; Weng et al. 2020). In particular, numerous mRNA vaccines are being developed to tackle infectious diseases and various types of cancer, with many advancing to different stages of clinical trials (Pardi et al. 2018).
Several features of in vitro transcribed mRNA contribute to its vaccine potential. First, the development process of an mRNA vaccine can be much faster than conventional protein vaccines (DeFrancesco 2017). In response to the pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2020, an mRNA vaccine was administrated to the first volunteer in a phase 1 clinical trial within ten weeks after the sequence of the viral genome was revealed (Lurie et al. 2020). Second, in vitro transcription reaction is easy to conduct, has a high yield, and can be scaled up (Pardi et al. 2018). Advanced industrial setup can manufacture mRNA up to kilogram scales (Versteeg et al. 2019). Third, mRNA vaccine enables the synthesis of antigen proteins in situ, eliminating the need for protein purification and long-term stabilization which are challenging for some antigens. Fourth, transportation and storage of mRNA may be easier than protein-based vaccines, since RNA, if protected properly against ribonucleases (RNases), is less prone to degradation compared to proteins (Stitz et al. 2017; Zhang et al. 2019). Because of these advantages, mRNA vaccines have great potential to be manufactured and deployed in a timely manner in response to rapid infectious disease outbreaks.
Despite mRNA’s appealing features and advances in the field, in vivo delivery of mRNA remains challenging. The first challenge is the instability of mRNA mostly due to enzymatic degradation by RNases. RNases are present ubiquitously throughout the body to degrade exogenous RNAs (Gupta et al. 2012). And mRNA, consisting of hundreds to thousands of nucleotides, has to reach the cytosol in full length for active translation. Hence, protection against RNases is critical for most in vivo delivery strategies. Secondly, efficient intracellular delivery of mRNA is another challenge owing to the negative charge and large size of mRNA molecules. The negative charge prevents most mRNA from translocating across the negatively charged cell membrane. The large size makes efficient encapsulation and delivery more challenging than other payloads, such as small molecules, siRNAs, and antisense oligonucleotides (ASOs). Various delivery strategies have been investigated to address these obstacles with different delivery materials, formulation methods, and routes of administrations.
The mRNAs used as vaccines can be categorized into conventional mRNAs and self-amplifying mRNAs. Conventional mRNAs are similar to endogenous mRNAs in mammalian cells, consisting of a 5’ cap, 5’ UTR, coding region, 3’ UTR, and a polyadenylated tail (Pardi et al. 2018; Kowalski et al. 2019). The typical size is 1–5 k nucleotides. When delivered to the cytosol, this type of mRNA is translated until its degradation without additional replication. On the other hand, self-amplifying mRNAs are derived from the genomes of single-stranded RNA viruses, such as alphaviruses (Brito et al. 2015). Besides encoding proteins of interest, self-amplifying mRNAs encode replication machinery consisting of several viral non-structural proteins (nsPs) to replicate themselves. Therefore, their typical size is approximately 8–12 k nucleotides, larger than the conventional mRNA vaccine. When delivered to the cytosol, self-amplifying mRNAs replicate themselves while expressing the designated proteins in a relatively large amount (Iavarone et al. 2017). More importantly, self-amplifying mRNAs are unique for vaccine applications because of their self-adjuvant nature (Maruggi et al. 2019). Many factors involved in their self-replication process, such as the double-stranded RNA (dsRNA) intermediate of replication (von Herrath and Bot 2003) and the nsPs in the replication machinery (Maruggi et al. 2013), could stimulate interferon-mediated immune responses (Pepini et al. 2017).
Three major types of proteins are encoded by mRNA vaccines: antigens (Grunwitz and Kranz 2017; Zhang et al. 2019), neutralizing antibodies (Stadler et al. 2017; Tiwari et al. 2018), and proteins with immunostimulatory activity (Bonehill et al. 2008; Manara et al. 2019). Antigens or neutralizing antibodies induce specific immune responses, while proteins with immunostimulatory activity, such as CD70 (Van Lint et al. 2012) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Manara et al. 2019) boost innate and/or adaptive immunity.
Advances in recent years made mRNA a promising vaccine platform. For example, chemical modifications of RNA using nucleotide analogs, such as pseudouridine, dramatically increased protein production in vivo by diminishing the translation inhibition triggered by the unmodified nucleotides (Kariko et al. 2008; Warren et al. 2010). High-performance liquid chromatography (HPLC) purification further increased the purity and translation capability of mRNA by removing the byproducts from in vitro transcription, such as dsRNA, which could induce inhibition of mRNA translation (Karikó et al. 2011; Weissman et al. 2013). Lipid and lipid-derived nanoparticles (LNPs) were previously used to deliver small molecule drugs and siRNAs (Brito et al. 2015; Ickenstein and Garidel 2019). The adaptation of LNPs for mRNA delivery greatly enhanced the delivery efficiency of mRNA both in vitro and in vivo (Dimitriadis 1978; Malone et al. 1989; Martinon et al. 1993). The use of new formulation technologies, such as continuous-flow microfluidic devices, enabled reproducible production of nanoparticles at various scales with controllable sizes (Jahn et al. 2008; Valencia et al. 2012).
In this chapter, we summarize the routes of administrations for mRNA vaccines, discuss mRNA delivery carriers and their corresponding formulation methods, and overview the challenges and future development of mRNA vaccines. A comprehensive overview of recent advances in mRNA vaccine delivery may facilitate the future development of novel delivery strategies and effective mRNA vaccines.
Read the full chapter here: Zeng C., Zhang C., Walker P.G., Dong Y. (2020) Formulation and Delivery Technologies for mRNA Vaccines. In: . Current Topics in Microbiology and Immunology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2020_217

