Correlation between the solid state of lipid coating and release profile of API from hot melt coated microcapsules
Solvent-free hot melt coating (HMC) provides a safer and more economic process compared to the conventional solvent coating techniques. However, drug release instability and the lack of fundamental understanding on it are limiting factors for application of HMC for industrial productions.
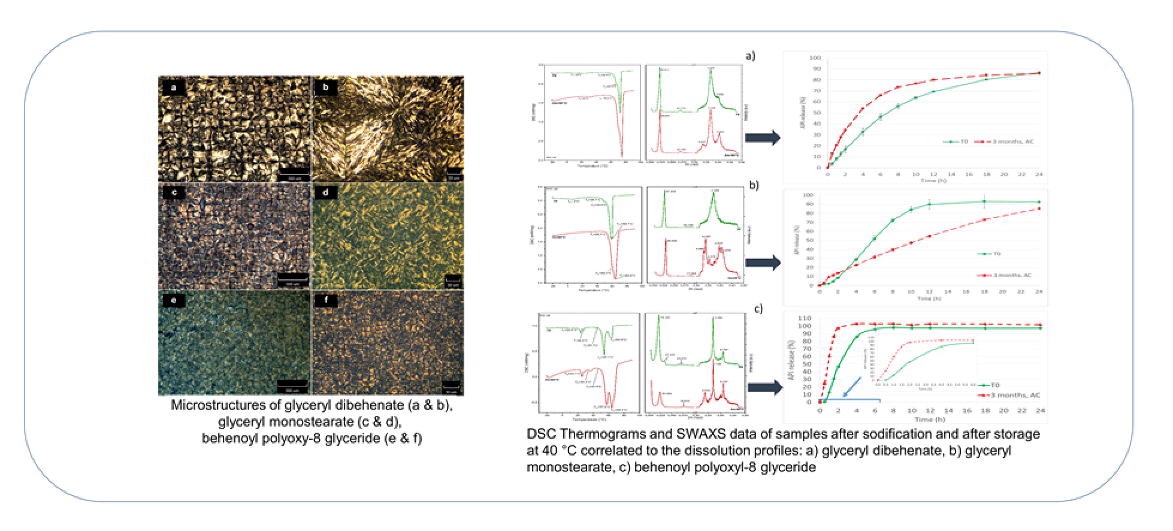
In this work, we investigated glyceryl dibehenate, glyceryl monostearate and behenoyl polyoxyl-8 glyceride as HMC materials. The microstructure and solid state alteration of lipids were studied via polarized light microscopy, DSC and powder x-ray diffraction. Microcapsules of N-acetylcysteine particles were provided with these excipients and stored under long term and accelerated conditions for 3 months.
The feasibility of selected lipids as HMC excipients was confirmed. The drug release from freshly coated microcapsules was dictated by microstructure, solid state and HLB of lipid coating. Alterations in the release profiles after storage under accelerated conditions were correlated with time-dependent structural alterations of selected lipids.
The faster drug release from glyceryl dibehenate and behenoyl polyoxyl-8 glyceride microcapsules was correlated with a low-melting small fraction composed by mixed phases in glyceryl dibehenate and the amorphous region of polyoxyl part in behenoyl polyoxyl-8 glyceride, respectively. The slower drug release from glyceryl dibehenate after storage was explained by the transition of lipid crystals to the β-form with dense crystalline structure.
The gained information can be used to design effective tempering strategies for providing stable pharmaceutical products. Read more on coating

