Predicting the critical quality attributes of ibuprofen tablets via modelling of process parameters for roller compaction and tabletting
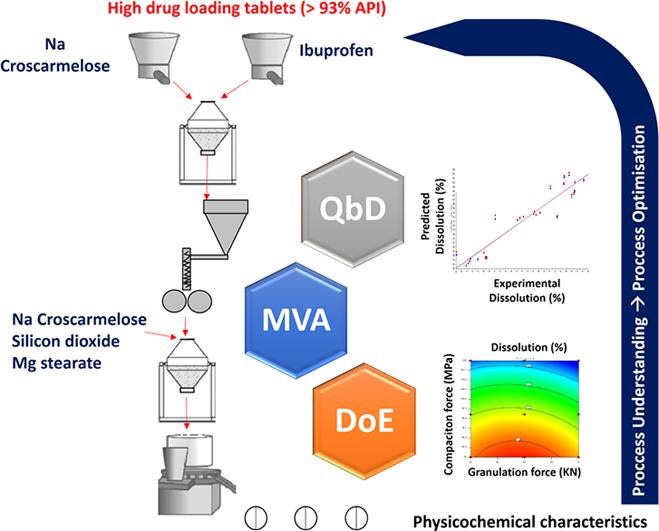
Roller compaction is a low cost granulation process which application is sometimes limited by the granular loss of compactability and reduced drug dissolution rate. Hence, the design of a robust manufacturing process is key in order to ensure quality of tablets.
In this study, for ibuprofen tablets with high drug loading (<7% excipients), the correlations between two critical process parameters (CPPs), namely roller force during granulation and compaction pressure during tabletting, and several critical quality attributes (CQAs) were investigated using a design of experiment (DoE) approach. Multivariate analysis (MVA) was utilized to identify the best regression model to predict CQAs such as disintegration, dissolution, weight uniformity, hardness, porosity and tensile strength for 200 and 600 mg ibuprofen tablets.
The tabletting compaction pressure had a greater impact on the aforementioned CQAs than compactor roller force. The Principal Component Analysis (PCA) correlation loading plot showed that compaction pressure was directly related to disintegration time, tensile strength and hardness, and inversely related to both the percentage of drug dissolved and porosity. The inverse correlations were observed for the roller force applied during dry granulation. Amongst all the regression models constructed, multiple linear regression (MLR) showed the best correlation between CPPs and CQAs. More on Ibuprofen tablets by roller compaction

