Additional information
| Manufacturer |
Meggle |
|---|---|
| Quality |
Compound of Maize Starch USP/NF, EP, JP, JP and Lactose Monohydrate USP/NF |
| Composition |
Compound of Maize Starch & Lactose Monohydrate |
| Dosage Form |
Capsule, coating and low dose formulations, Tablets |
| Function |
capsule filling, cores for coating and low dose formulations, Filler-binder for direct compression and dry granulation processes. Also used for fast-dissolving tablets |


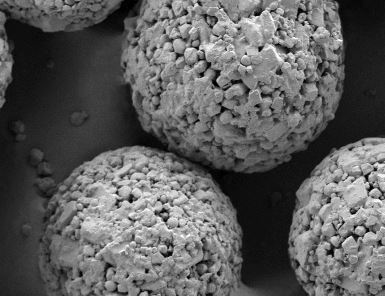

 Particle size distribution batch-to-batch consistency of StarLac® by air-jet-sieving.Data obtained from a permanent in-process-controll (IPC) of subsequent batches over a time period of 12 months.
Particle size distribution batch-to-batch consistency of StarLac® by air-jet-sieving.Data obtained from a permanent in-process-controll (IPC) of subsequent batches over a time period of 12 months.