Biopharmaceutical Understanding of Excipient Variability on Drug Apparent Solubility Based on Drug Physicochemical Properties. Case Study: Superdisintegrants
The presence of different excipient types/brands in solid oral dosage forms may affect product performance and drug bioavailability. Understanding the biopharmaceutical implications of superdisintegrant variability (changes in material properties), variation (changes in excipient amount) and interchangeability (use of different excipient types with the same intended functionality) in oral drug performance would be beneficial for the development of robust final dosage forms.
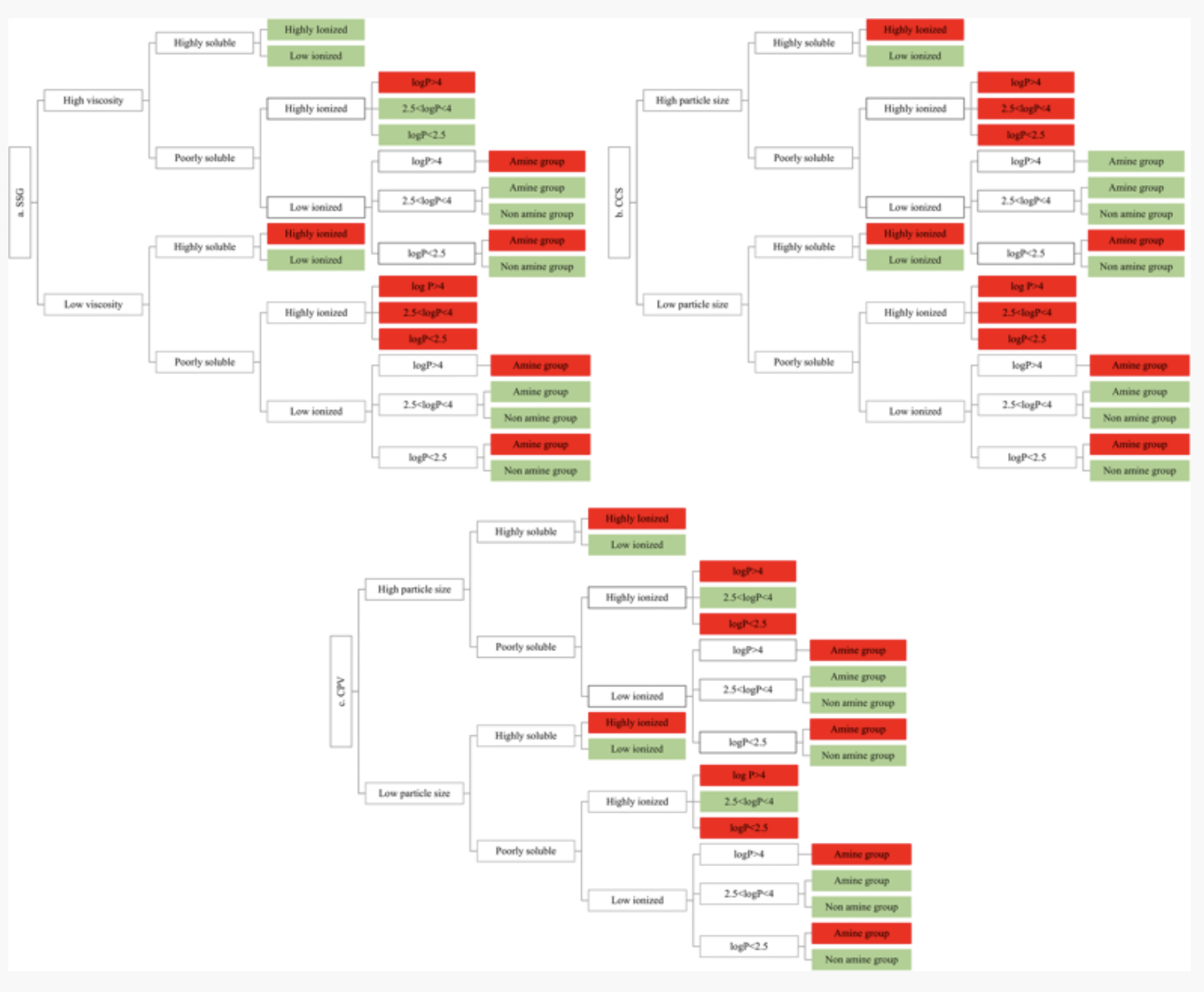
The current study investigated the impact of superdisintegrants (sodium starch glycolate, croscarmellose sodium, crospovidone) on the apparent solubility of drugs with different physicochemical properties (drug ionisation, drug lipophilicity, drug aqueous solubility). Compendial and biorelevant media were used to assess the impact of gastrointestinal conditions on the effects of excipient on drug apparent solubility. For the majority of compounds, changes in drug apparent solubility were not observed in superdisintegrant presence, apart from the cases of highly ionised compounds (significant decrease in drug solubility) and/or compounds that aggregate/precipitate in solution (significant increase in drug solubility).
Excipient variability did not greatly affect the impact of excipients on drug apparent solubility. The use of multivariate data analysis identified the biopharmaceutical factors affecting excipient performance. The construction of roadmaps revealed that superdisintegrants may be of low risk for the impact of excipients on oral drug performance based on drug solubility alone; superdisintegrants activity could still be a risk for oral bioavailability due to their effects on tablet disintegration. Download the publication as pdf by clicking on the image


