Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders
The treatment of various central nervous system (CNS) diseases has been challenging, despite the rapid development of several novel treatment approaches. The blood–brain barrier (BBB) is one of the major issues in the treatment of CNS diseases, having major role in the protection of the brain but simultaneously constituting the main limiting hurdle for drugs targeting the brain. Nasal drug delivery has gained significant interest for brain targeting over the past decades, wherein the drug is directly delivered to the brain by the trigeminal and olfactory pathway. Various novel and promising formulation approaches have been explored for drug targeting to the brain by nasal administration. Nanoemulsions have the potential to avoid problems, including low solubility, poor bioavailability, slow onset of action, and enzymatic degradation. The present review highlights research scenarios of nanoemulsions for nose-to-brain delivery for the management of CNS ailments classified on the basis of brain disorders and further identifies the areas that remain unexplored. The significance of the total dose delivered to the target region, biodistribution studies, and long-term toxicity studies have been identified as the key areas of future research.
Download the full article here: Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders
or continue reading here: Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230.
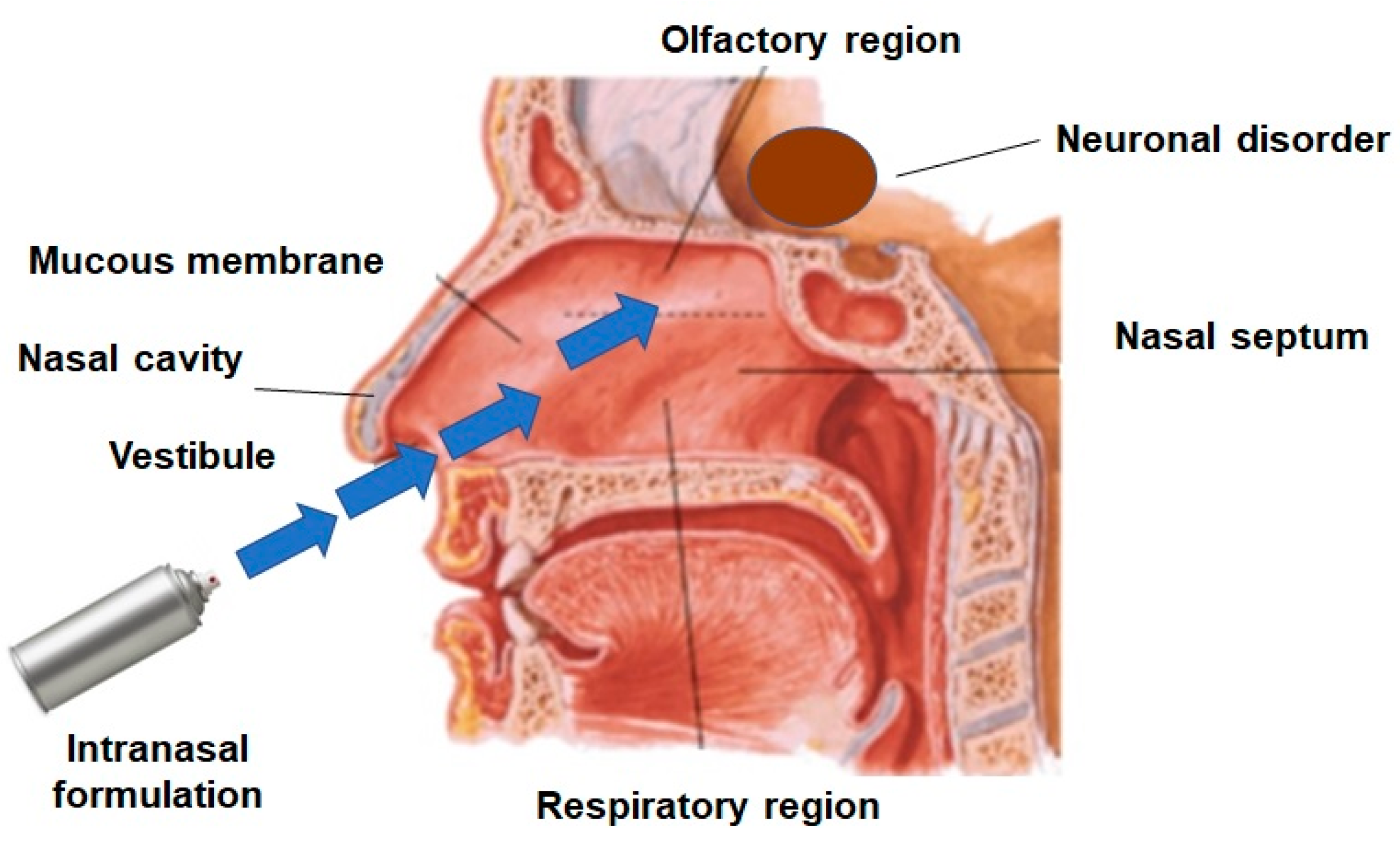
Nasal administration of molecules active in the brain
Learn more about the Nasal drug delivery platform MetP

Components of nanoemulsions mentioned in the publication
3.1.1. Oils
The major problem of new molecular entities in the drug discovery and development pipeline is their poor water solubility, which affects several key properties of therapeutic agents, such as the pharmacokinetic and pharmacodynamic parameters. Hence, oils are used for the development of NE to achieve the maximum solubility of drugs. The lipophilicity of oils is directly proportional to the solubility of drugs [32]. The solubilizing capacity of oils decreases in the order of vegetable oils > medium-chain triglycerides > medium-chain mono- and diglycerides [33]. Further, the solubility of drugs also depends on the concentration of oils in the NE formulations. The globule size of NE increases with an increase in the oil concentration [34]. At the same time, a larger globule size reduces drug permeation from the nasal mucosa. Some oils have permeation-enhancing properties as well. NEs showed selectivity in the uptake of some drugs, such as linolenic acid, polyunsaturated and omega-6 fatty acids, and pinolenic acid [35]. Edmond et al. proved that oleic acid with one cis-double bond did not get transported across the BBB, while linoleic acid with two cis-double bonds and 18 monocarboxylic acids efficiently entered the brain after intranasal administration [36].
The maximum solubility of a drug can be achieved by striking a correct balance between the concentration of the emulsifying agent (surfactant) and oils. Here, one needs to select the region having maximum emulsification in the phase diagram [34]. Better drug permeation can be achieved through a minimum globule size; hence, formulations having higher globule sizes have less permeation through the nasal mucosa. Several oils have significant permeation-enhancing properties through the nasal mucosa. For example, NE of quetiapine fumarate containing butter oil showed significant nose-to-brain delivery [37]. It was deduced that polar lipids from butter oil enhanced the permeation through the nasal mucosa.
3.1.2. Surfactants
Surfactants are essential components of NE formulation and signify an important role in the surface tension reduction. Surfactants stabilize NE by preventing the phase separation and coalescence of globules. Further, surfactants affect solubilization of the drugs and increase the permeation of drugs through the nasal mucosa due to alterations of the fluidity and damage to the tight junctions of epithelial layers [38]. Several studies report that the globule size of NEs decreases with increasing the concentrations of the surfactants. The lower the globule size, the better the permeation and, hence, the drug concentration in the brain. Nevertheless, the structural integrity of the nasal mucosa is critically affected by surfactants. Hence, the surfactant concentration should be selected carefully, keeping in view the safety considerations of the nasal mucosa [39,40].
3.1.3. Cosurfactants
Surfactants alone are not able to reduce the surface tension to the desired level, because most of the surfactants used in the development of NEs are single-chain surfactants. Therefore, cosurfactants are incorporated to achieve the desired hydrophilic-lipophilic balance (HLB) [41,42]. Cosurfactants increase the fluidity of the formulations by reducing the interfacial tension, which can facilitate emulsification and stabilize the NE. For the development of stable NE, a judicious combination of surfactant and cosurfactant is crucial. The construction of ternary-phase diagrams is a commonly used methodology to optimize the working range and the optimum concentration(s) of oil, surfactant, and cosurfactant. An increase in the concentration of the cosurfactant deceases the globule size of the NE, and, ultimately, the drug concentration will be enhanced. Some most commonly used cosurfactants in the development of NEs for intranasal administration are transcutol-P, butan-1-ol, chiral alcohols, sorbitol, and polyethylene glycol [33].

