Novel Cleaning-in-Place Strategies for Pharmaceutical Hot Melt Extrusion
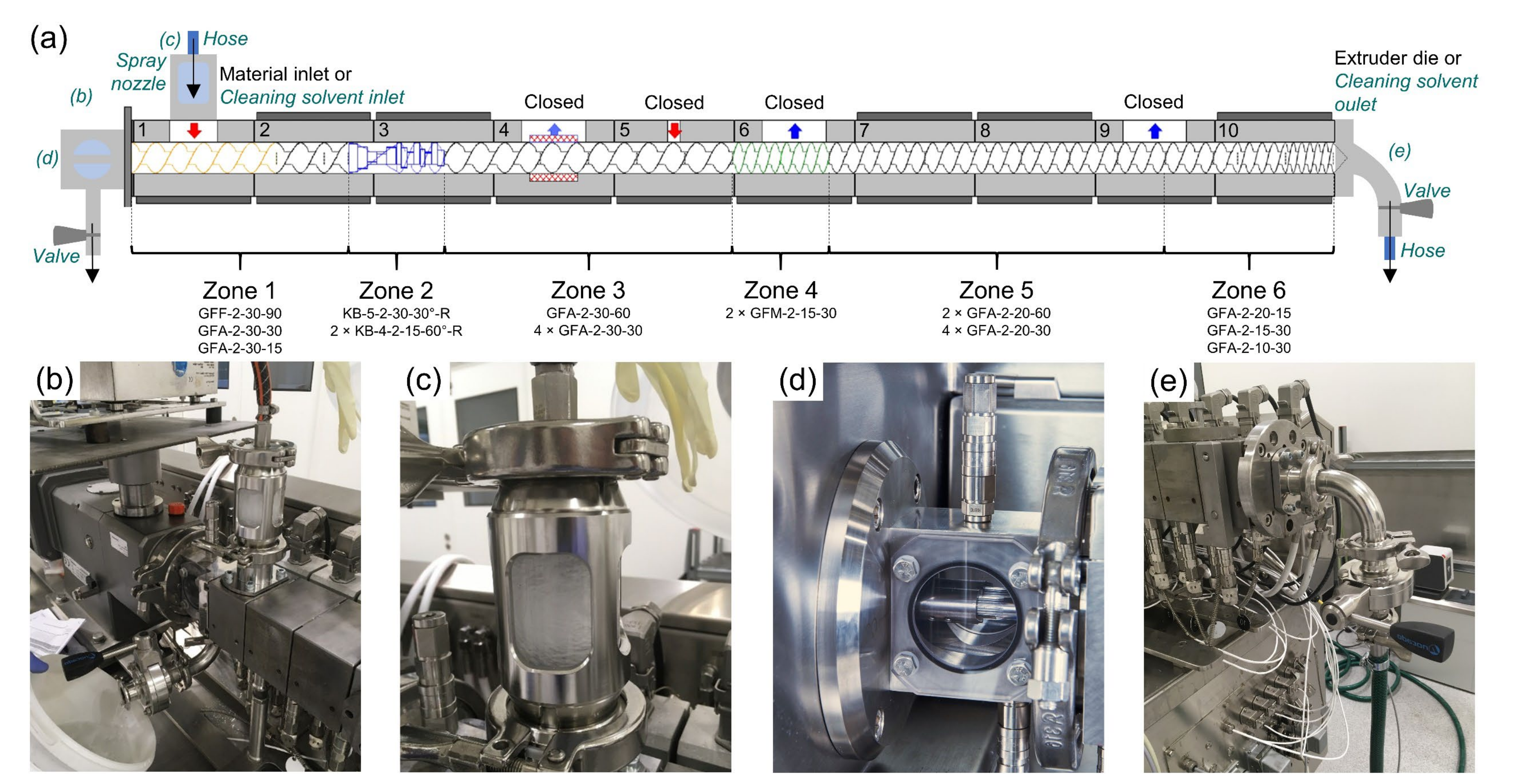
To avoid any type of cross-contamination, residue-free production equipment is of utmost importance in the pharmaceutical industry. The equipment cleaning for continuous processes such as hot melt extrusion (HME), which has recently gained popularity in pharmaceutical applications, necessitates extensive manual labour and costs. The present work tackles the HME cleaning issue by investigating two cleaning strategies following the extrusion of polymeric formulations of a hormonal drug and for a sustained release formulation of a poorly soluble drug. First, an in-line quantification by means of UV–Vis spectroscopy was successfully implemented to assess very low active pharmaceutical ingredient (API) concentrations in the extrudates during a cleaning procedure for the first time. Secondly, a novel in-situ solvent-based cleaning approach was developed and its usability was evaluated and compared to a polymer-based cleaning sequence. Comparing the in-line data to typical swab and rinse tests of the process equipment indicated that inaccessible parts of the equipment were still contaminated after the polymer-based cleaning procedure, although no API was detected in the extrudate. Nevertheless, the novel solvent-based cleaning approach proved to be suitable for removing API residue from the majority of problematic equipment parts and can potentially enable a full API cleaning-in-place of a pharmaceutical extruder for the first time.
Download the full MDPI publication: Novel Cleaning-in-Place Strategies for Pharmaceutical Hot Melt Extrusion
Spoerk, M.; Koutsamanis, I.; Matić, J.; Eder, S.; Patricia Alva Zúñiga, C.; Poms, J.; Urich, J.A.A.; Andreína Lara García, R.; Nickisch, K.; Eggenreich, K.; Berghaus, A.; Reusch, K.; Relle, Y.; Khinast, J.; Paudel, A. Novel Cleaning-in-Place Strategies for Pharmaceutical Hot Melt Extrusion. Pharmaceutics 2020, 12, 588.
EASY CLEANING OF HOT MELT EXTRUSION
HME Cleaner Plus (GMP)
More than half of New Chemical Entities (NCEs), which were developed recently, tend to show high crystallinity and poor solubility in water. Solid dispersion is a technique to improve the solubility and bioavailability of these NCEs. Hot-melt extrusion (HME) is one of the main methods to prepare
solid dispersions.
Enteric Polymers (e.g. AQOAT®, EUDRAGIT®, Kollicoat® etc.), which are widely used as an enteric coating agent, are useful carriers for solid dispersions and applicable for HME. However, these polymers are insoluble in water and users often have difficulties cleaning their extruder after processing.
HME Cleaner Plus (GMP) is an almost water-soluble purge compound with a micro-cleansing effect which solves this cleaning difficulty for the HME process. HME Cleaner Plus (GMP) provides effective clean-up because of its high detergency and low residual properties.
Shin-Etsu developed the HME Cleaner in 2012 as technical material. Based on Shin-Etsu’s formulation, BIOGRUND now offers this cleaner, produced in accordance with IPEC GMP guidelines and meeting all requirements of USP/ NF, Ph.Eur. and JP. All ingredients have been used as pharmaceutical excipi- ents including water-soluble cellulose ethers.
How to use HME Cleaner Plus (GMP)
After your actual extrusion of solid dispersion, set your extruder (mixing zones) at more than 160°C (depending on scale size). HME Cleaner Plus (GMP) may adhere to the barrels when it is below 160°C. In cases of extrusion at higher temperature (200°C), keep the temperature for the first extrusion with HME Cleaner Plus (GMP) until substitution is completed. Then adjust the tempera- ture (170°C) for additional cleaning extrusion. HME Cleaner Plus (GMP) starts to decompose over 200°C.
Keywords: cleaning verification; cleaning-in-place; hot melt extrusion; API contamination; UV–Vis spectroscopy; process analytical technology; swab and rinse tests; estradiol; estriol; ibuprofen; Ethylene-vinyl acetate copolymer; Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonioethyl methacrylate chloride); Eudragit RL-PO; HME Cleaner Plus, hydroxypropyl methylcellulose, methylcellulose, propylene glycol, amorphous silicon dioxide

