Colon-targeted 3D-Printed mesalamine tablets: Core-shell design and in vitro/ex-vivo evaluation
Abstract
Objectives
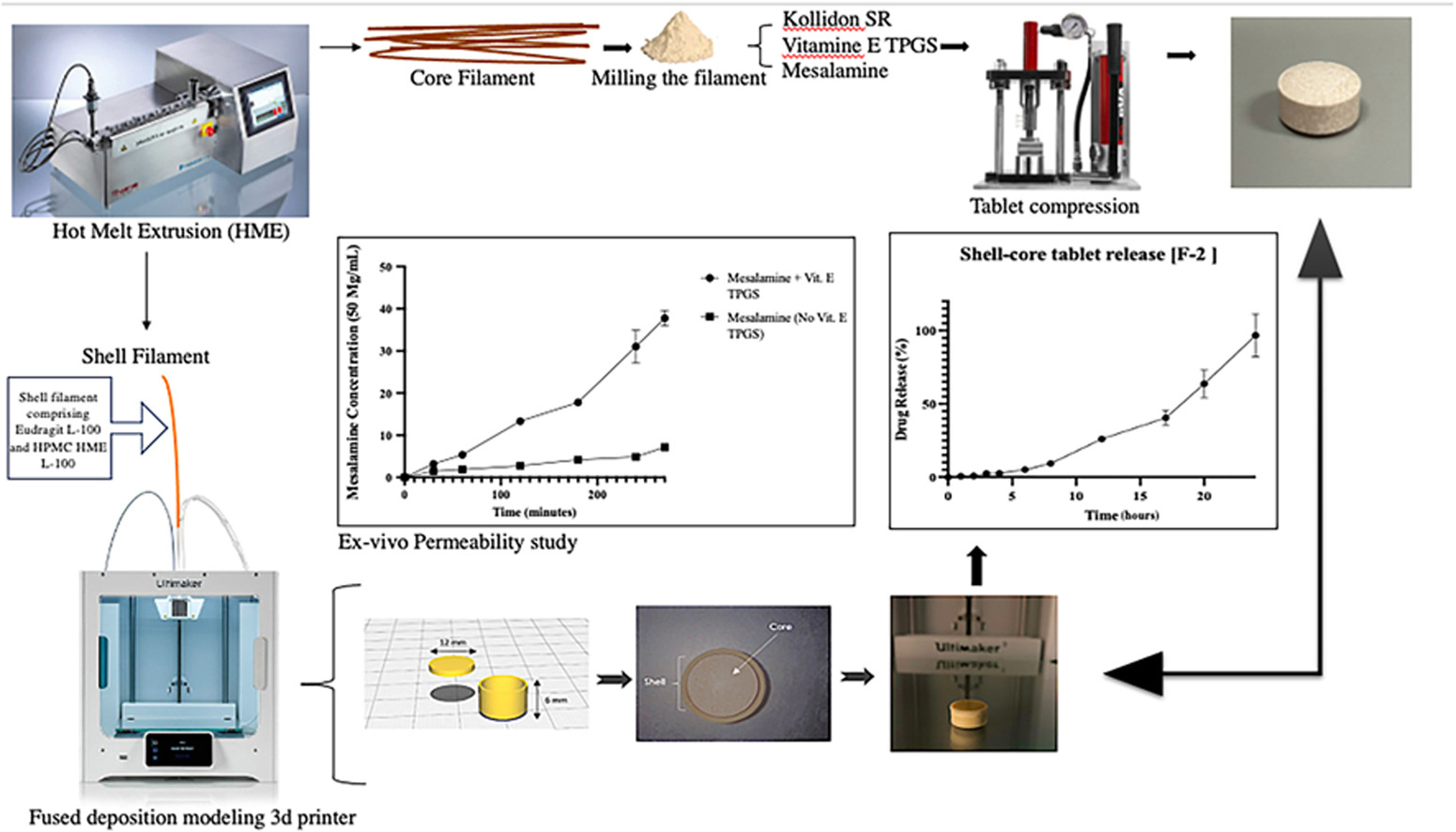
The present study is intended to develop a shell-core tablet using a hot melt extrusion (HME)-based dual-nozzle fused deposition modeling (FDM) three-dimensional (3D) printing approach. The primary objective was to establish a sustained-release colonic drug delivery system and improve mesalamine’s permeability by incorporating Vitamin E TPGS as a permeation enhancer.
Method
The study utilized Kollidon® SR for sustained release, L-100, and HPMC HME L100 for the tablet’s protective shell. Six filament formulations were tested, and the mechanical properties of the shell filaments, including the three-point bending, Hooke’s law, and stiffness, were assessed. Drug release profiles of the tablets were evaluated using the USP-II dissolution apparatus, and permeability characteristics were gauged using the non-everted intestinal sac method. Solutions containing 5% w/v mesalamine and 2.5% w/v Vitamin E TPGS were employed, with pure mesalamine as a control.
Highlights
- The core composition of the tablet included an extended-release polymer (Kollidon SR) and permeability enhancer (Vitamin E TPGS) with the drug (Mesalamine) to extend the drug release and enhance drug absorption.
- Shell composition contains dual polymer; Eudragit L-100 for pH-sensitive release in the colon at pH above 6 and HPMC HME L-100 for gel formation to prevent release in the upper GI tract.
- Shell filament produced via hot-melt extrusion (HME) and 3D printed to ensure protection of the core tablet.
Results
Optimal filament ratios were identified as 50:50 for the core and 30:70 for Eudragit L-100 to HPMC HME L100 for the shell. The resulting tablets achieved a prolonged drug release of up to 24 hours for the core. They ensured minimal drug release in the upper gastrointestinal tract (∼5% in the first 5 hours), effectively targeting the colon. Incorporating Vitamin E TPGS led to a 3.6-fold increase in mesalamine absorption compared to the control, and the addition of Kollidon® SR notably improved the flow properties of Mesalamine powder.
Conclusion
In conclusion, this innovative approach has the potential to achieve a colon-specific drug delivery system.
Read more here
Materials
Mesalamine was purchased from Fisher Scientific (Hanover Park, IL, USA). Methacrylic acid copolymer (Eudragit® L100) was donated by Degussa-Röhm Co. (Darmstadt, Germany). Affinisol HPMC HME 100LV (molecular weight = 180 kDa) was donated by Colorcon Inc. (PA, USA). Kollidon® SR and Vitamin E TPGS (d-α-tocopheryl polyethylene glycol succinate) were gifted from BASF (Ludwigshafen, Germany). Avicel® 102 (microcrystalline cellulose) was obtained from the FMC Biopolymer.
Nouf D. Alshammari, Ahmed Almotairy, Mashan Almutairi, Peilun Zhang, Esraa Al Shawakri, Sateesh Kumar Vemula, Michael A. Repka, Colon-targeted 3D-Printed mesalamine tablets: Core-shell design and in vitro/ex-vivo evaluation,
Journal of Drug Delivery Science and Technology, 2024, 105580, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105580.
CPHI & PMEC China will be held at 19-21 June 2024. Register for free:



