Anti-counterfeiting protection, personalized medicines − development of 2D identification methods using laser technology
Counterfeiting of the products for healing is as old as trading, and it is difficult to quantify the magnitude of the problem. It is known that substandard and/or falsified (SF) medicines are a growing global threat to health, and they cause serious social and economic damage. The EU has a strong legal framework for medicines, it is mandatory to meet the requirements of Directive 2011/62/EU.
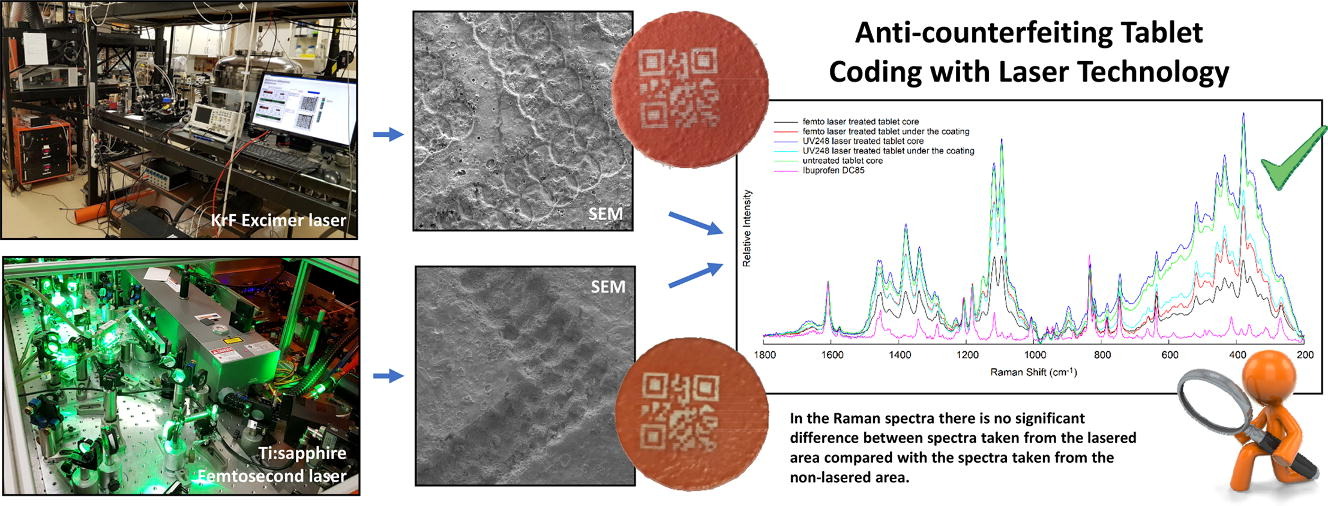
Serialisation prevents SF medicinal products from entering the legal distribution chain. The present study is an extension of the original idea and aims to develop a laser technology-based method to mark an individual traceable code on the surface of the tablet, which technology can also be used for marking personalized medicines. The method is based on the ablation of the upper layer of a double-layer, differently coloured coating. The 2D code should be formed without harming the functional layer, and anyone with a smartphone integrated with a camera should be able to authenticate these drugs with a suitable application.
The present findings confirmed that KrF excimer laser and Ti:sapphire femtosecond laser are efficient and reliable for marking. These should be promising candidates for pharmaceutical companies that would like to have additional protection against drug counterfeiters.
Read the full open access article here
More about microcrystalline cellulose
Tablet core and coating materials
The active ingredient of the model tablet was Ibuprofen DC 85 (Ibu, BASF, Germany) 16.66% (w/w), and excipients: microcrystalline cellulose (Vivapur 102, JRS Pharma, Germany) 74.33% (w/w), crospovidone (Kollidon CL-M, BASF, Germany) 5% (w/w), talc (Molar Chemicals, Hungary) 3% (w/w) and magnesium-stearate (Molar Chemicals, Hungary) 1% (w/w) were used as received.
The shape of the tablets was as follows: Nr1 type: round with flat surface and no break line on it (diameter: 12 mm, crown height: 4.1 mm, average weight: 600 mg). Nr 2 type: round with flat surface and no break line on it (diameter: 10 mm, crown height: 3.1 mm, average weight: 300 mg). Nr 3 type: round with flat surface and no break line on it (diameter: 10 mm, crown height: 4.8 mm, average weight: 500 mg).
Placebo tablets were also used as coating aid for reasons of saving time and material. The placebo tablets were round with biconvex surface and had no break line on them (diameter: 10 mm, crown height: 4.2 mm, average weight: 350 mg).
The first (functional) film-forming agent was an aqueous-based enteric coating dispersion: Eudragit L30 D55® (Evonik Nutrition & Care, Germany). The second film-forming substance was a hydroxypropyl–methylcellulose (HPMC)-based coating formula, Sepifilm PW Red (Seppic S.A., France)
Conclusion
During laser ablation, it is important not to cause any change in the medicine. Commonly, it is the thermal effect which can cause problems in the material’s quality. At the same time, the coating has to be removed at specific points. This can be achieved by choosing the right laser and setting the optimal parameters.
It can be concluded that in the experiment, the threshold exceeded the ablation threshold of titanium dioxide during lasering, and it was sufficient in its removal. Titanium dioxide did not interfere with QR code recognition, which was performed with a mobile phone.
It was found that ablation with UV248 laser and Femto laser did not cause a qualitative change in the material during laser marking. The UV248 laser is a laser for laboratory use, while Femto lasers are commonly used in the industry. However, the higher repetition rate of the Femto laser allows faster and more efficient coding, which has key importance in the production. Furthermore, the results show that due to the high performance in the fs pulse length, the wavelength is no longer a critical parameter as it is for ns or longer pulses. The thermal effects are negligibly low in the fs region even at high peak powers. The thermal effects of laser ablation can be avoided by reducing the wavelength or the impulse length based on the current study.
It is known that the efficient use of this technology requires further development in speed. Other high energy near-infrared pulse lasers that operate at multi-kHz versions (with a repetition frequency of multi ten-kHz and also MHz), could potentially further shorten the ablation time. Those devices could even be used for line speed marking in pharmaceutical companies. Also, these devices are scalable in energy and they are capable of implementing the one-shot technique. Further research is needed in this direction by testing new lasers and new techniques.
In conclusion, we propose a functionally advanced marking by the lasers mentioned in this article, highlighting the Femto laser as a solution for pharmaceutical companies that would like to have additional protection against drug counterfeiters or to label personalized medicines, knowing that the technique needs further improvement.
Article information: Krisztina Ludasi, Orsolya Jójárt-Laczkovich, Tamás Sovány, Béla Hopp, Tamás Smausz, Attila Andrásik, Tamás Gera, Zsolt Kovács, Géza Regdon jr. Anti-counterfeiting protection, personalized medicines − development of 2D identification methods using laser technology, International Journal of Pharmaceutics, 2021. https://doi.org/10.1016/j.ijpharm.2021.120793.

