Advances in antimicrobial polymeric iodophors
Abstract
Despite centuries of research on this chemical element, the ability of iodine to form complexes with water-soluble polymers and various other molecules remains a topic of great interest. The fascination with iodine and iodophors arises from their remarkable antimicrobial properties against a wide range of pathogens. This review encompasses historical information on iodine and iodophors, showcases existing iodine-containing products available in the market, explores the diverse physicochemical methods employed to study polymer-iodine complexes, and assesses recently published studies on iodophors. Recent advances in this field include the use of new experimental techniques to get new insights into the mechanisms of complexation and structure of these complexes; preparation of iodophors using novel polymers, nano- and micro-particles; and fabrication of antimicrobial surfaces capable of slow iodine release.
Introduction
Iodine is a nonmetallic chemical element that belongs to the halogen family. It was discovered by Courtois in 1811 [1] and was named as a new chemical element by Gay-Lussac in 1813 [2]. At room temperature the molecular iodine (I2) is a bluish-black solid with crystalline appearance and ability to sublime forming a deep violet vapor irritant to mucosal surfaces [3].
Iodine has strong antiseptic properties which was first discovered by Davaine in 1873 [4] and paved the way for its numerous biomedical applications. It is currently well-documented that iodine has bactericidal, fungicidal, virucidal and sporicidal activities [5]. Antimicrobial properties of iodine continue to attract attention of researchers even in the recent decade due to the strong need for combating various infections and preventing their spreading.
The antiseptic applications of iodine expanded substantially with the discovery of its ability to form complexes with some hydrophilic polymers and smaller molecules such as cyclodextrins and surfactants. Nowadays these formulations are commonly called iodophors. The first reaction of this type was reported by Colin and de Claubry in 1814 [6] for the complexes of molecular iodine with starch. This reaction has found numerous applications in analytical chemistry due to the rapid change in colour from yellow/brown typical for solutions of molecular iodine to a dark blue-black of the starch-iodine complex [7]. Some starch-iodine complexes also found applications as antiseptics in wound care. Iodophors can also be formed by reaction of molecular iodine with some synthetic water-soluble polymers. One of these iodophors is formed through the complexation between poly(N-vinylpyrrolidone) (Povidone) and molecular iodine, which was discovered by Shelanski in 1949 [8], [9].
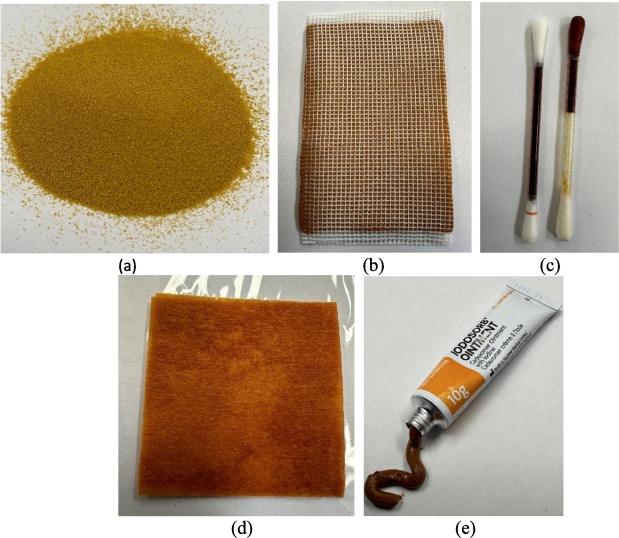
Currently, iodophors are widely used as antiseptic products for disinfection of surfaces, wound treatment, pre- and post-operative care, mouth washes, gargles, and nasal sprays. They may be formulated as solutions, gels, sprays, powders, wound dressing pads and cotton sticks. Fig. 1 shows some examples of commercial iodophors.
Despite over two centuries of research into iodine and iodophors, many fundamental aspects of the complexes formed by this halogen and hydrophilic polymers are still poorly understood. This review aimed to present an analysis of the progress in this field from the physicochemical properties of iodine to the development of modern methods to study polymer-iodine interactions and structure of their complexes, and to the biomedical applications of these systems.
Table 1: Characteristics of some polymer-iodine complexes.
| Polymer | Mechanism of complexation | Color of polymer-iodine complex |
|---|---|---|
| Polyvinyl alcohol (PVA) | Inclusion of iodide ions into helical PVA chains | Red, however, if boric acid is added the product becomes blue |
| Poly(N-vinylpyrrolidone) (PVP) | Formation of donor–acceptor complex | Brown |
| Polyethylene Glycol (PEG) | Formation of charge transfer complex between with I3 - ions and oxygen atoms | Non-specific color (Depends on concentration of iodine) |
| Poly(2-ethyl-2- oxazoline) (PEOZ) | Monomolecular complexation between PEOZ chains and iodine species with conformational changes | Yellow |
| Starch | Left-handed helical structure with a polyiodide chain | Amylose forms blue complex and amylopectin forms red one |
| Chitosan | Interaction of hydroxyl groups in D-glucosamine moiety in chitosan with primarily I3 - ions | Non-specific color at room temperature and purple after freezing |
| Cellulose derivatives | Sandwich structure formed by a pair of neighboring cellulose chains and triiodide species. | Non-specific color |
| Pectin | Charge transfer complexation with participation of the carbonyl and hydroxyl groups of pectin and absorption of I3 - ions, I2 and In - polyiodide ions. | The color of complexes can change from red to black depending on the concentration of the polymer and iodine |
Download the full article as PDF here Advances in antimicrobial polymeric iodophors
or read it here
Danelya N. Makhayeva, Galiya S. Irmukhametova, Vitaliy V. Khutoryanskiy, Advances in antimicrobial polymeric iodophors, European Polymer Journal, Volume 201, 2023, 112573, ISSN 0014-3057, https://doi.org/10.1016/j.eurpolymj.2023.112573.
Read also following article about “antimicrobial” here:
- Lyophilized Lipid Liquid Crystalline Nanoparticles as an Antimicrobial Delivery System
- Novel long-acting brimonidine tartrate loaded-PCL/PVP nanofibers for versatile biomedical applications: fabrication, characterization and antimicrobial evaluation
- Triggered release of antimicrobial peptide from microneedle patches for treatment of wound biofilms