Shin-Etsu AQOAT® – Enteric coating polymer and solid dispersion carrier

Shin-Etsu supplies the various excipients for pharmaceutical industry. Let us introduce you Shin-Etsu AQOAT® Hypromellose acetate succinate (HPMCAS, NF, JP), an enteric coating material that can be used in aqueous or organic media.
Hypromellose is a non-toxic material which has been used in pharmaceutical, food, and cosmetic industries for many years. Based on hypromellose, acetyl and succinoyl groups are introduced to the hydroxyl groups of the backbone, and this constitutes Shin-Etsu AQOAT®, hypromellose acetate succinate (HPMCAS).
There are nine grades with different particle sizes (fine, medium, granular) and chemical substitution levels of acetyl and succinoyl groups to obtain an opening pH range from 5.5 to 6.5.
Shin-Etsu AQOAT® is one of the leading polymers in solid dispersion technology. It is suitable for spray-drying, hot melt extrusion, co-precipitation and other techniques. There are more than 14 approved products in the US using Shin-Etsu AQOAT® in solid dispersion.
Shin-Etsu AQOAT® Hypromellose Acetate Succinate, NF, JP
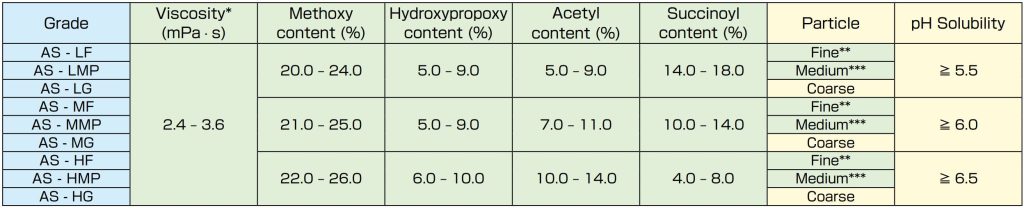
Features:
- For enteric coating, various coating methods can be applied such as aqueous, organic solvent, ammonia neutralized and dry coating. Coating methods can be selected depending on the characteristic of API. For example, dry coating is suitable for water and solvent sensitive API.
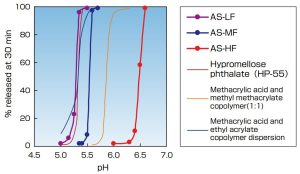
- Riboflavin granules were coated with various enteric coating agents. P e r c e n t r e l e a s e o f riboflavin at 30 minutes was measured in USP Phthalate buffer (〜pH 5.6) and USP Phosphate buffer (pH 5.8〜)
- Shin-Etsu AQOAT® is also used in solid dispersion for solubility and bioavailability enhancement.
In order to prepare solid dispersion, various methods are applicable such as spray dry, spray coating, hot melt extrusion (HME), coprecipitation etc. As Shin-Etsu AQOAT® can dissolve into various organic solvents and has relatively low glass transition temperature (Tg), it is one of the most suitable polymers in solid dispersion. Numerous scientific papers reported that Shin-Etsu AQOAT® was able to enhance the drug solubility more effectively compared to other polymers.

Tg was determined by DSC experiment under the following test condition;
Equipment: DSC Q2000 (TA Instruments, Japan), Heating rate: 10ºC/min,
Referred to the second heating run N2 gas atmosphere Sample size: 3 mg
See the full brochure on “Pharmaceutical Excipients by Shin-Etsu” here
(find more on Shin-Etsu AQOAT® on page 3, click the picture to download the brochure)
Source: Shin-Etsu, brochure “Pharmaceutical Excipients by Shin-Etsu”




