Best practices in dealing with novel excipients
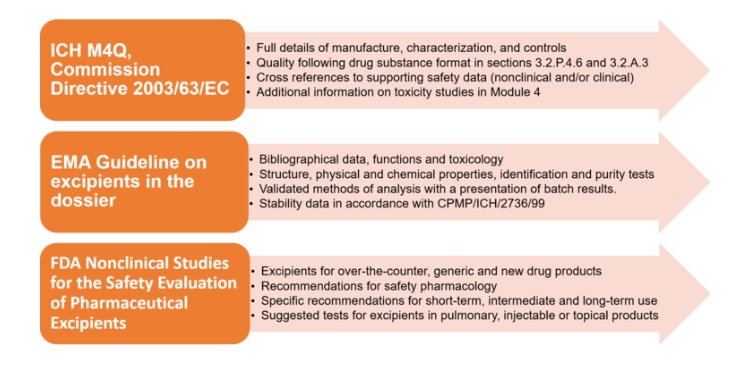
Novel excipients play a crucial role in bringing new, improved and safer drugs to the pharmaceutical market. There are however significant challenges in the development of innovative excipients, mainly because of the absence of globally aligned regulatory mechanisms. Biopharma Excellence has successfully supported several projects involving novel excipients. In this article, we provide an overview of the regulatory landscape and strategic considerations for the development of novel excipients in the EU and US.
Excipients are the main components of any pharmaceuticals’ formulation and crucial for its safe and effective use. The functionality of excipients is very diverse: They can be used as fillers, lubricants, coloring matters, antioxidants, preservatives, adjuvants, stabilizers, emulsifiers, solubilizes, permeation enhancers, etc. Although the majority of approved products only contain traditional, well-studied compendial excipients, the use of novel excipients is unavoidable for the formulation of some advanced products. Also, progressively more companies prefer to develop sophisticated and innovative formulations involving novel excipients. This opens the way for advanced drug delivery systems aimed to improve the therapeutic profile of drug substances or to meet the need for more convenient routes of administration.
What is a novel excipient?
ICH, EMA: A novel excipient is an excipient which is being used for the first time in a drug product, or by a new route of administration. It may be a new chemical entity or a well-established one which has not yet been used for human administration and /or for a particular human administration pathway.
FDA: New excipients are any inactive ingredients that are intentionally added to the therapeutic and diagnostic products, but that: Continue reading on novel excipients here

