Cannabidiol safety considerations: Development of a potential acceptable daily intake value and recommended upper intake limits for dietary supplement use
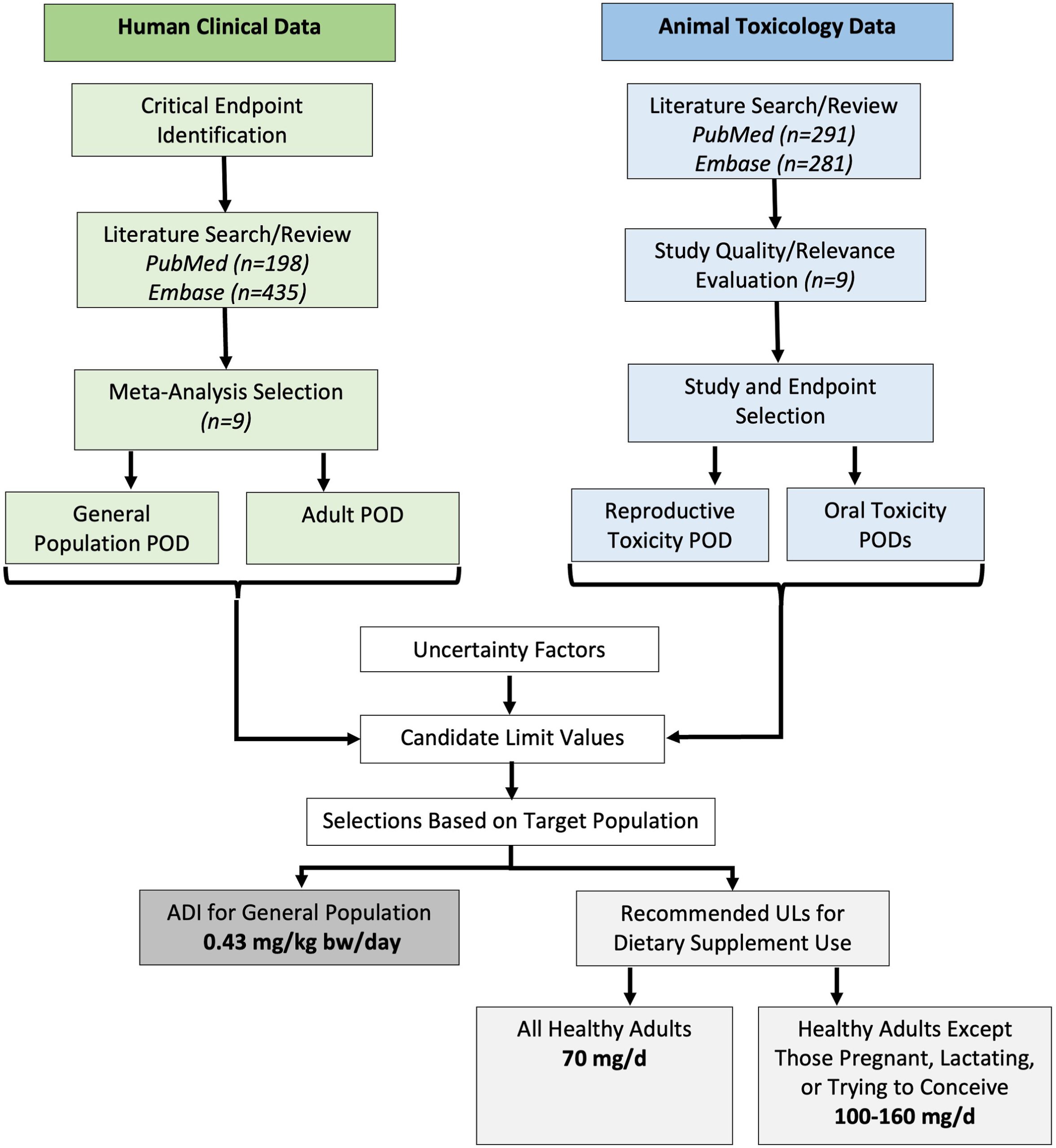
Consumer use of hemp-derived products continues to rise, underscoring the need to establish evidence-based safety guidance. The present study sought to develop recommendations for oral upper intake limits of cannabidiol (CBD) isolate. Sufficiently robust and reliable data for this purpose were identified from published human clinical trials and guideline-compliant toxicity studies in animal models.
Highlights
Potential Acceptable Daily Intake value of 0.43 mg/kg-bw/d was derived for the general population.
ADI applies to sensitive subpopulations, including children, and from all sources, including food.
Upper Intake Limit (UL) of 70 mg/d was developed for dietary supplement use by all healthy adults.
ULs of 100 and 160 mg/d derived for healthy adults not trying to conceive, pregnant, or lactating.
Recommendations are based solely on the authors’ assessments and are not regulatory guidelines.
Based on the metrics used in this assessment, a potential Acceptable Daily Intake (ADI) value of 0.43 mg/kg-bw/d (e.g., 30 mg/d for 70-kg adult) was determined for the general population based on liver effects in human studies. This value applies to the most sensitive subpopulations, including children, over a lifetime of exposure and from all sources, including food. For dietary supplements with adequate product labeling intended for use by healthy adults only, a potential Upper Intake Limit (UL) of 70 mg/d was determined based on reproductive effects in animals. For healthy adults, except those trying to conceive, or currently pregnant or lactating, a conservative dietary supplement UL of 100 mg/d was identified based on liver effects; however, as the target population excludes individuals at risk for liver injury, an alternative dietary supplement UL of 160 mg/d for this population can also be considered.
1. Introduction
Worldwide use of hemp-derived consumer products continues to rise, despite a lack of consistent safety-related guidance or regulatory oversight. Hemp, typically defined as Cannabis sativa L. containing ≤0.3% delta-9-tetrahydrocannabinol (THC) on a dry-weight basis, contains more than 120 identified cannabinoids, as well as an array of terpenes and phenolic compounds (AHPA, 2022; EC, 2013; Rupasinghe et al., 2020; Walsh et al., 2021). A large number of pre-clinical and clinical safety studies have been conducted with the most common non-intoxicating cannabinoid in hemp, cannabidiol (CBD). In the United States (US) and Canada, a survey of 45,300 adults (age 16 years and older) demonstrated that 16.2–26.1% had used CBD-containing products in the previous twelve months (Goodman et al., 2022). Similarly, the Brightfield Group (2023) has estimated that 15% of Americans (49.8 million) use CBD regularly, reporting consumption levels ranging from ≤20 mg/day and ≥1000 mg/day. The amount of CBD consumption associated with consumer products varies by individual; for example, in one survey conducted in the United Kingdom (UK), participants reported using between ≤24 mg/day and ≥200 mg/day of CBD (Moltke and Hindocha, 2021). Continued consumer interest in these products has been attributed to perceived beneficial effects on conditions such as anxiety, pain, depression, and insomnia, as well as for well-being, relaxation, and stress relief (Goodman et al., 2022; Moltke and Hindocha, 2021; Fortin et al., 2021; Corroon and Phillips, 2018). The clinical evidence for potential therapeutic effects of CBD has been reviewed in recent publications, such as O’Sullivan et al. (2023). In addition to CBD isolate, many consumer products are hemp extracts containing a mixture of cannabinoids and terpenes, where CBD typically comprises a large fraction of the ingested material (e.g., 5–90% CBD). Thus, determining safe levels of CBD intake in dietary supplements, foods, and/or beverages is critical to ensuring consumer safety.
Despite the amount of safety-related data on CBD, acceptable daily intake (ADI) values have not yet been established by any regulatory agency or authoritative body. An ADI is intended to apply to the general population, which includes all age groups (including children, typically 12 weeks of age and older), physiological states, and pregnant and lactating individuals (IPCS, 1987, 2020). The value is defined as the estimated amount of a substance, expressed on a body-weight basis, that can be ingested daily over a lifetime without appreciable health risk (EFSA, 2012a; IPCS, 2004).
While no regulatory-based guidance for consumption of CBD from foods or supplements by children has been established, recommended upper intake levels for adults using CBD-containing novel foods and/or dietary supplements have been established for CBD by the UK Food Safety Authority (FSA, 2023), Health Canada (2022), and the Australian Therapeutic Goods Administration (TGA, 2021). Conversely, the US Food and Drug Administration (FDA, 2022, FDA, 2023) and the European Food Safety Authority (EFSA, 2022a) have concluded the currently available data to be insufficient for this purpose, citing uncertainties in the dataset, such as a need for additional data on long-term exposure, and potential effects on liver and reproductive toxicity.
Despite the general absence of harmonized authoritative positions, consumer use of CBD and other hemp-derived products continues to grow, thus creating an immediate need to establish evidence-based recommendations for intake to enable continued safe use of CBD-containing products. A recent increase in relevant published literature provides a sufficient basis from which to accomplish this critical need. This new information further supplements the large body of human CBD clinical trial data that already exists (reviewed in Arnold et al., 2023; Chesney et al., 2020; Lo et al., 2023; Souza et al., 2022). In addition, new guideline-compliant genotoxicity, subchronic oral toxicity, and reproductive toxicity studies in animal models have recently been published which help to address data gaps highlighted previously by regulatory agencies (Henderson et al., 2023a, 2023b, 2023c; Tallon and Child, 2023).
The goal of the current study was to use a systematic approach to review the publicly available safety data to develop recommendations for oral consumer use of hemp-derived CBD isolate. Specifically, we attempted to derive: 1) a potential ADI for the general population and from all sources, and 2) recommended upper intake limits (ULs) for dietary supplement use by healthy adults.
Download the full study as PDF here: Cannabidiol safety considerations: Development of a potential acceptable daily intake value and recommended upper intake limits for dietary supplement use
or read it here
Rayetta G. Henderson, Melissa Vincent, Brianna N. Rivera, Marcel O. Bonn-Miller, Candace Doepker, Cannabidiol safety considerations: Development of a potential acceptable daily intake value and recommended upper intake limits for dietary supplement use, Regulatory Toxicology and Pharmacology, Volume 144, 2023, 105482, ISSN 0273-2300,
https://doi.org/10.1016/j.yrtph.2023.105482.

