Overcome Challenges of Elastic Deformation with the Right Excipient – Case Study With Captopril by MEGGLE

Abstract
Developing a medicine in which the active ingredient has elastic deformation is always challenging, as elastic forces affect the compression capacity of the formulation, resulting in tablets with capping, low tablet breaking force and high friability. Finding an ideal formulation with excipients that can mask the effects of elastic deformation is part of obtaining a tablet with good tablet properties so that the manufacturing process has quality and robustness.
Among the active ingredients that have elastic deformation is Captopril, an antihypertensive, sold in solid pharmaceutical form. Conventional Captopril formulations are often unable to mask the effects of elastic deformation, which can cause significant implications during production and also on the effectiveness of the medicine.
Objective
MEGGLE´s study aims to present the obstacles encountered in conventional Captopril formulations and highlight how lactose-based technological excipients can promote improvement and scaling more effective and robust.
Materials and Methods
Three formulations have been designed for these studies. The blends were blended by a Turbula Mixer T2F (Willy A. Bachofen AG Maschinenfabrik), with a 2 L vessel, running at 42 rpm. Mixing time was 15 minutes for all the components and additional 3 minutes after adding the lubricant.
The tablets were produced by direct compression using a pilot scale rotary tableting machine Piccola–D (Riva SA – Argentina), controlled by the software “The Director®“ (SMI – USA). Compression was performed using a gravimetric feeder. For the placebo tablets, a 7 mm circular, concave punch was used, stettings of machine were adjusted to obtain tablets of 130 mg.
The machine operated at 20 rpm and 50 rpm and compression force of 5 kN, 10 kN, 15 kN and 20 kN to verify the behavior of each formulation under different process parameters.
(click table to enlarge)

Formulation F.1 represents a conventional formulation with FlowLac® 100.
Formulation F.2 represents the physical admixture of CombiLac®, which has the same concentrations of lactose, microcrystalline cellulose and maize starch as present in CombiLac® using FlowLac® 100.
Formulation F.3 represents a formulation with a co-processed excipient of 70 % alpha-lactose, 20 % microcrystalline cellulose and 10 % corn starch, CombiLac®.
The comparison between formulation F.2 and F.3 aims to present the differences in results between co-processing by spray-drying and a physical mixture.
Tablet Weight / Weight Variation (USP <2091>)
20 tablets were individually weighed in an analytical scale and the average weight and the deviation coeficient were determined.
Tablet Breaking Force (USP <1217>)
10 tablets were investigated employing an ERWEKA TBH 125 hardness tester.
Tablet Disintegration (USP <701>)
6 tablets were investigated in terms of disintegration according to the USP method, using water at 37 °C and an ETHIK disintegration apparatus.
Tablet Friability (USP <1216>)
20 tablets were dedusted and weighed (w1), submitted to an ETHIK friability test apparatus according to USP-NF at 25 rpm for 4 minutes. At the end, tablet weight was checked again after dedusting of the tablets (w2).
- Process with parameters / Experimental Set-up
- Preparation of the data acquisition/ data acquisition
- Documentation
Results and Discussion
5.1 Tablet Compression
During the manufacturing process, all formulations showed excellent flow, which allowed the powder to flow freely through the funnel and properly filling the dies.
However, due to the elastic deformation of the Captopril molecule, laminated tablets were seen when increasing the speed from 20 rpm to 50 rpm and the compression force from 10 kN to 15 kN in formulation F.1. The same was repeated in formulation F.2 when the compression force was increased by 15 kN and 20 kN at both speeds, something that in formulation F.3 only happened at 20 kN and 50 rpm.
The compression process showed that despite the excellent flow and compressibility properties of the excipients, a conventional formulation, such as F.1 and F.2, are not capable of dealing with the characteristics of elastic forces and for this reason, problems such as capping were seen.
5.2 Tablet Weight
The weight of the tablets and the weight variation were investigated with the aim of verifying the ability of the excipients in the formulation to maintain homogeneity in different process parameters.
In all three formulations, excellent flow was observed from FlowLac® 100 and CombiLac®, which provided a low weight variation and in no test were tablets weighing outside the USP specification observed.
The smallest weight variation was seen in formulation F.3 (CombiLac®) of 1.1 %, followed by formulation F.1 (FlowLac® 100) of 1.7 % and 2.5 % of formulation F.2.
5.3 Tablet Breaking Force
The tablet hardness test results were illustrated in the graph below:
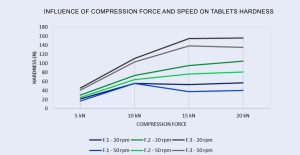
As seen in the graph, formulation F.1, which represents a conventional formulation, had the greatest influence of elastic deformation forces on the Captopril molecule, in addition to having lower tablet breaking force when compared to formulation F.2 and F.3, formulation F.1 suffered a drop in tablet breaking force when reaching 15 kN of compression force. With 20 kN of compression force at 20 rpm F.1 achieves 57 N of breaking force and drops 29 % when the speed is increased. Comparing with F.3, CombiLac provides a formulation with 155.9 N at 20 rpm and 135.2 N at 50 rpm.
On the other hand, the formulation with F.3, CombiLac®, despite a small drop in tablet breaking force when reaching 20 kN and 50 rpm of 2.3 %, was the formulation with the highest tablet breaking force. When comparing the F.3 formulation, CombiLac® and the F.2 formulation, CombiLac® PAM (FlowLac® 100), the co-processed CombiLac® was able to provide a higher tablet breaking force of 48.19 % at 20 rpm and 67.53 % at 50 rpm compared to the physical compared to the physical mixture CombiLac® PAM.
Conclusions and Recommendations
According to the results obtained in this study, it was possible to identify that Captopril consists of a molecule with elastic deformation. This particular property of Captopril causes problems like tablet capping during production, high friability and variation in tablet breaking force.
Therefore, choosing the correct excipients to mask these effects was extremely important and necessary to guarantee a robust manufacturing process. At the end of the study, it was possible to confirm that conventional formulations have greater difficulty in maintaining adequate properties.
It was shown with the right choice of excipient it is possible to overcome these difficulties and to work in a very robust manufacturing process. In this case study two excipients were used to compare the performance of Captopril, normal spray-dried lactose and a co-processed excipient, combining lactose monohydrate (70 %), MCC (20 %) and native corn starch (10 %). As a highlight, it could be shown that the formulation with CombiLac® was able to achieve excellent mechanical stability of the tablets, even at low compression forces. High tablet breaking force, no or only low friability and no capping tendency were seen. The disintegration time was also excellent.
This case study illustrates the robustness and quality that CombiLac® can promote for developments with difficult active ingredients and that it is a perfect fit to ease processes during up-scaling.
See the full case study on “Case Study With Captopril” here
(click the picture to download the brochure)
Source: MEGGLE case study “Case Study With Captopril”, website Case Study Captopril: Overcome Challenges of Elastic Deformation (meggle-pharma.de)


