Decoding the Small Size Challenges of Mini-tablets for Enhanced Dose Flexibility and Micro-dosing
Mini-tablets are an age appropriate dosage form for oral administration to pediatric and geriatric patients, either as individual mini-tablets or as composite dosage units.
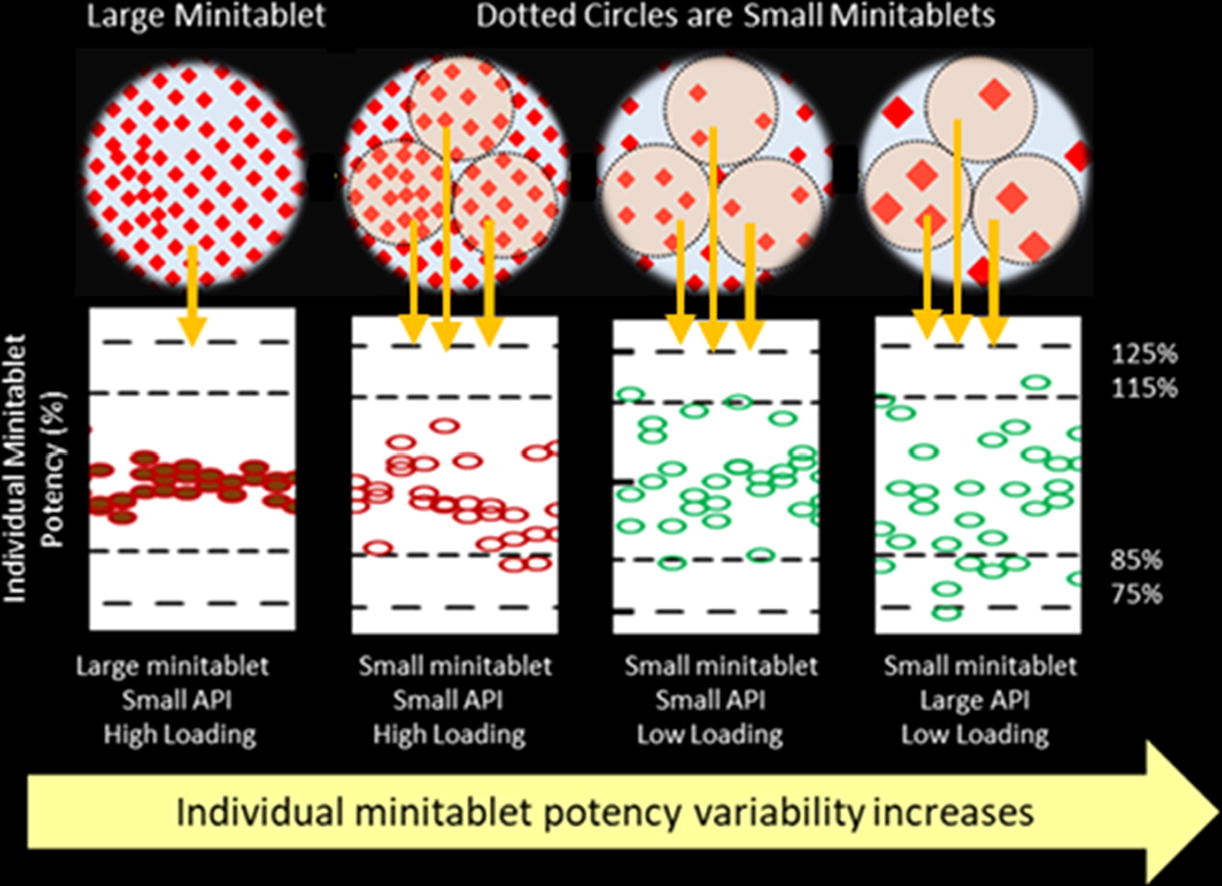
Smaller mini-tablets than the commonly used 2 mm or larger size would offer added advantages to more tailored delivery of micro-dose. This work demonstrated a drug substance particle size, loading range and mini-tablet size range to achieve acceptable quality attributes of mini-tablets. A platform formulation with 60, 80, and 100 μm (particle size D6,3) ibuprofen at 3, 14, and 25% loadings were directly compressed into 1.2, 1.5, 2, and 2.5 mm diameter mini-tablets.
With an enhanced weight control approach, all the mini-tablet batches except the 1.2 mm mini-tablets with 100 µm ibuprofen at 3% loading would achieve acceptable content uniformity as individual mini-tablets (USP <905> L2 criteria) and as composite dosage units of five or more mini-tablets (USP <905> L1 criteria). A dissolution method was developed and successfully utilized to evaluate the formulations herein. Small size mini-tablets, small ibuprofen particle size, and low dose (or low ibuprofen loading) enhanced the dissolution.
In addition, hypothetical scenarios of potential dose flexibility, dose range, dose titration, and excipient burden were discussed. The results of this study provide guidance for development of smaller size mini-tablets that enable dosing as a single or composite dosage unit, reduce excipient burden and leverage dispensing technology to achieve enhanced dosing flexibility and micro-dosing. More on mini tablets

