Effect of Compaction Pressure on the Enzymatic Activity of Pancreatin in Directly Compressible Formulations
1. Introduction
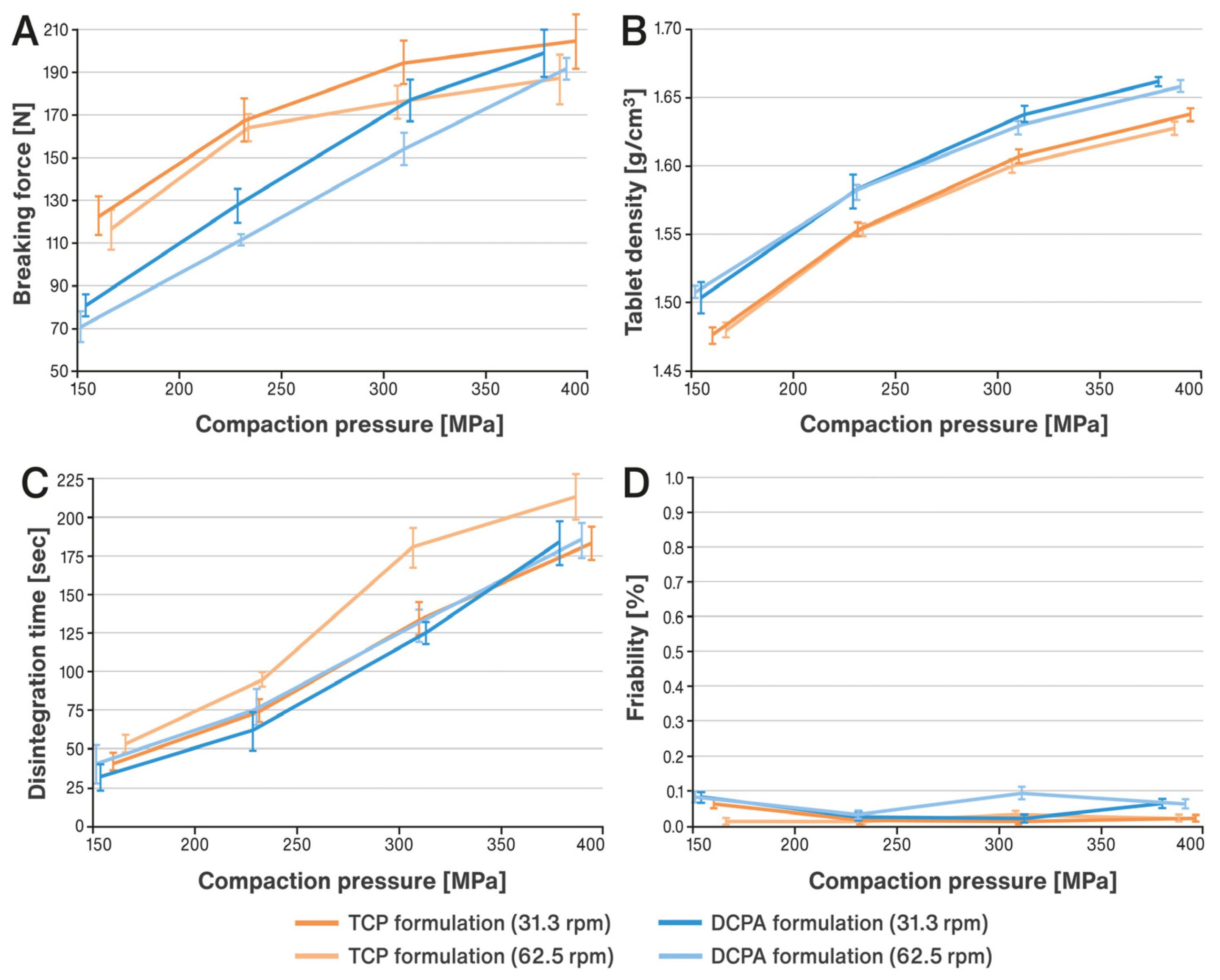
2. Materials and Methods
Download the full study as PDF here: Effect of Compaction Pressure on the Enzymatic Activity of Pancreatin in Directly Compressible Formulations
or read it here
Zakowiecki, D.; Edinger, P.; Hess, T.; Paszkowska, J.; Staniszewska, M.; Romanova, S.; Garbacz, G. Effect of Compaction Pressure on the Enzymatic Activity of Pancreatin in Directly Compressible Formulations. Pharmaceutics 2023, 15, 2224.
https://doi.org/10.3390/pharmaceutics15092224

