One Step In Situ Co-Crystallization of Dapsone and Polyethylene Glycols during Fluidized Bed Granulation
Abstract
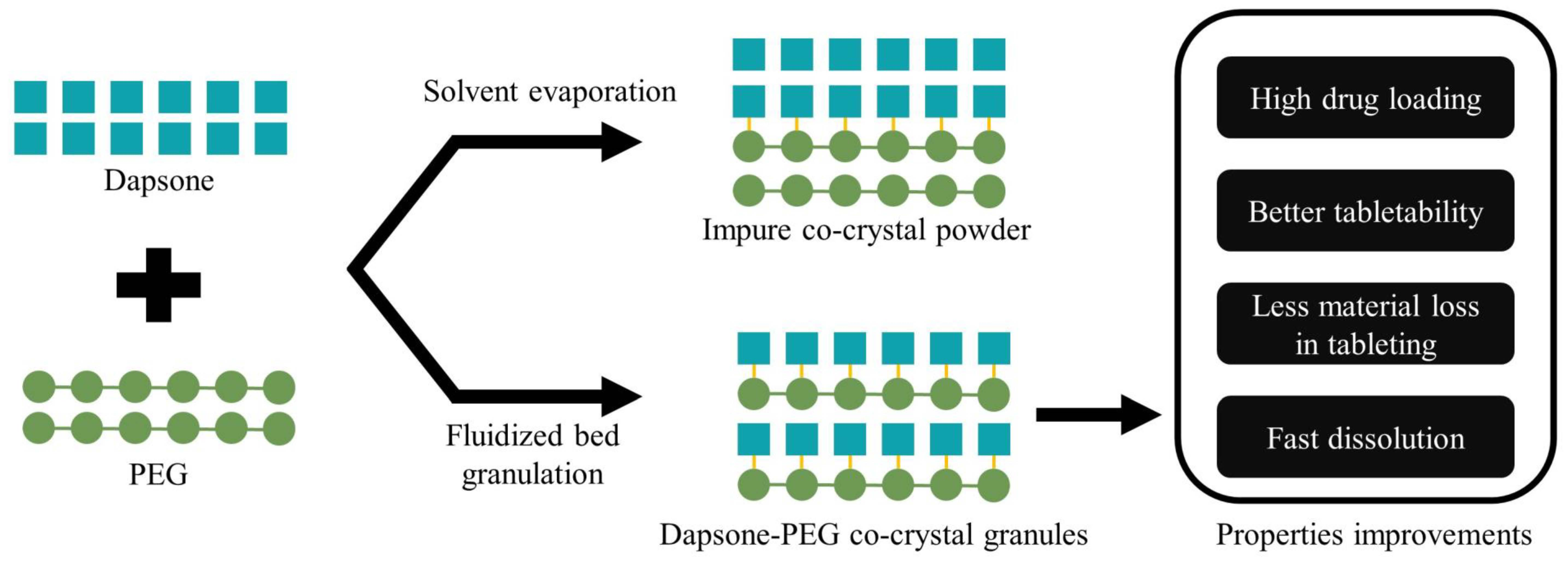
Several studies have demonstrated the feasibility of in situ co-crystallization in different pharmaceutical processes such as spray drying, hot melt extrusion, and fluidized bed granulation (FBG) to produce co-crystal-in-excipient formulations. However, no previous studies have examined such a one step in situ co-crystallization process for co-crystal formulations where the coformer is a polymer. In the current study, we explored the use of FBG to produce co-crystal granules of dapsone (DAP) and different molecular weight polyethylene glycols (PEGs). Solvent evaporation (SE) was proven to generate DAP-PEGs co-crystals at a particular weight ratio of 55:45 w/w between DAP and PEG, which was subsequently used in FBG, using microcrystalline cellulose and hydroxypropyl methyl cellulose as filler excipient and binder, respectively. FBG could generate co-crystals with higher purity than SE. Granules containing DAP-PEG 400 co-crystal could be prepared without any additional binder. DAP-PEG co-crystal granules produced by FBG demonstrated superior pharmaceutical properties, including flow properties and tableting properties, compared to DAP and DAP-PEG co-crystals prepared by SE. Overall, in situ co-crystallization via FBG can effectively produce API-polymer co-crystals and enhance the pharmaceutical properties.
Introduction
Today, oral delivery is still one of the most popular choices for drug delivery due to high patient acceptability and compliance compared to other delivery routes. According to a recent study, oral solid dosage (OSD) forms of pharmaceuticals comprise around 90% of the global pharmaceutical product market, and 60% of small molecule active pharmaceutical ingredients (APIs) are designed into OSD formulations [1]. However, designing suitable OSD formulations is still challenging because the absorption of APIs from the gastrointestinal (GI) tract is a complex process and will be impacted by the API’s properties such as dissolution rate, solubility, permeability, and physicochemical stability [2,3,4,5].
The Biopharmaceutics Classification System (BCS) is a well-established system to evaluate APIs’ properties for OSD formulation design. The BCS involves categorization of APIs into four classifications according to their solubility and intestinal permeability. Both properties are the key factors affecting drug absorption. Approximately 40% of the drugs on the market and 90% of drugs in the development phase are troubled by their low solubility [6].
Co-crystals constitute a well-studied solid form that shows the potential to improve various pharmaceutical properties of APIs, in particular solubility and dissolution rate [7,8]. The Food and Drug Administration (FDA) has provided a definition for co-crystals since 2018, whereby, co-crystals are crystalline materials composed of two or more different molecules, typically API and co-crystal formers (“coformers”), in the same crystal lattice [9]. The crystal lattice of co-crystals is normally composed of two or more different small (low molecular weight) molecules. The co-crystal coformer is either a small molecule excipient, normally from the Generally Recognized as Safe (GRAS) list or another small molecule API (a drug–drug co-crystal) [10,11]. Compared to the well-studied API–small molecule co-crystals, the study of API–polymer co-crystals is still an area that has not been fully explored and exploited. Moreover, polymeric crystalline inclusion complexes (CICs) are liable to be confused with API–polymer co-crystals.
The clear differences between these two solid forms have been well explained by Chappa et al. Firstly, a characteristic of co-crystals is strong hydrogen bonding between the different molecules, but in CICs, there is a polymer inclusion in the API’s crystal channel, with weak or no hydrogen bond interaction. Secondly, a single unique endothermic melting behavior can be observed for co-crystals, but a dissociation event after API melting can be observed for CICs [12,13,14]. Compared to well-studied small molecule co-crystals, only a few cases of polymer co-crystals have been identified and characterized, but the APIs concerned are not delivered orally [15,16,17,18]. However, the more recent discovery of dapsone–polyethylene glycol (DAP-PEG) co-crystals has highlighted the emerging subset of polymer co-crystals in pharmaceutical co-crystals [14], which brings much more opportunities and options for pharmaceutical formulation scientists to enhance an API’s physicochemical properties. Dapsone is a BCS class II API and DAP-PEG600 co-crystal was shown to have a higher solubility than DAP in different physiological pH conditions [14].
Aside from discovering novel co-crystals, challenges of how to scale up the production of co-crystals and how to develop a uniform, stable, and well-behaved crystalline solid dispersions (CSDs) of co-crystals in a one step in situ co-crystallization process are gaining increasing attention from formulation scientists. The normal approaches used for generating or discovering co-crystals at small scale include neat milling [19,20], liquid-assisted milling [21], and solvent evaporation (SE) [22], while scale-up processing techniques for in situ co-crystallization, such as spray drying, hot melt extrusion, and fluidized bed processing, are gaining popularity. All three of these scale-up techniques have been proven to generate pure co-crystals either alone or together with suitable excipients [23,24,25,26]. Furthermore, there is already a growing number of studies that investigate how different excipients in such one-step production processes affect the co-crystal formulation properties, such as crystallinity, dissolution, rate of dissociation, tabletability, etc. [26,27,28,29]. Nevertheless, to the best of our knowledge, there are no studies to date that investigate the production of polymeric co-crystals in such one step in situ co-crystallization processes.
In this study, we focused on the previously reported DAP-PEGs co-crystals [14] as examples of polymeric co-crystals to assess if formulations of polymeric co-crystals can be generated in a one step in situ co-crystallization process employing fluidized bed granulation (FBG). The objectives of this study were (1) to determine whether formulations containing polymer co-crystals can be obtained by FBG, (2) to investigate the possibility of the polymer co-former of such co-crystals acting as a binding agent, and (3) to assess whether the tablets made from co-crystal granules produced by FBG have better tabletability than equivalent physical mixtures (PMs) and if the dissolution performance of tablets prepared from such granules is better than equivalent PMs.
Download the full article as PDF One Step In Situ Co-Crystallization of Dapsone and Polyethylene Glycols during Fluidized Bed Granulation
or read more here
Materials
Dapsone (DAP form III) was purchased from Glentham Life Sciences (Corsham, UK). PEG 400, PEG 4000, and PEG 6000 were purchased from Tokyo Chemicals Incorporated (Zwijndrecht, Belgium). PEG 600, hydrochloric acid (HCl), and acetone were purchased from Sigma Aldrich (Arklow, Ireland). PEG 1000, PEG 1500, and methanol were purchased from Thermo Fisher Scientific (Dublin, Ireland). Microcrystalline cellulose (MCC) (Avicel PH 102) was a gift from DuPont (Cork, Ireland). Hydroxypropyl methylcellulose (HPMC) (27–30% methoxy content and 7–12% hydroxypropoxy content, 40–60 cp, around 22,000 g/mol)) was purchased from Alfa Aesar (Ward Hill, MA, USA).
Shao, S.; Bonner, D.; Twamley, B.; Singh, A.; Healy, A.M. One Step In Situ Co-Crystallization of Dapsone and Polyethylene Glycols during Fluidized Bed Granulation. Pharmaceutics 2023, 15, 2330. https://doi.org/10.3390/pharmaceutics15092330
Read more on Binder – Pharmaceutical Excipients here:


