Effects of semaglutide-loaded lipid nanocapsules on metabolic dysfunction-associated steatotic liver disease
Abstract
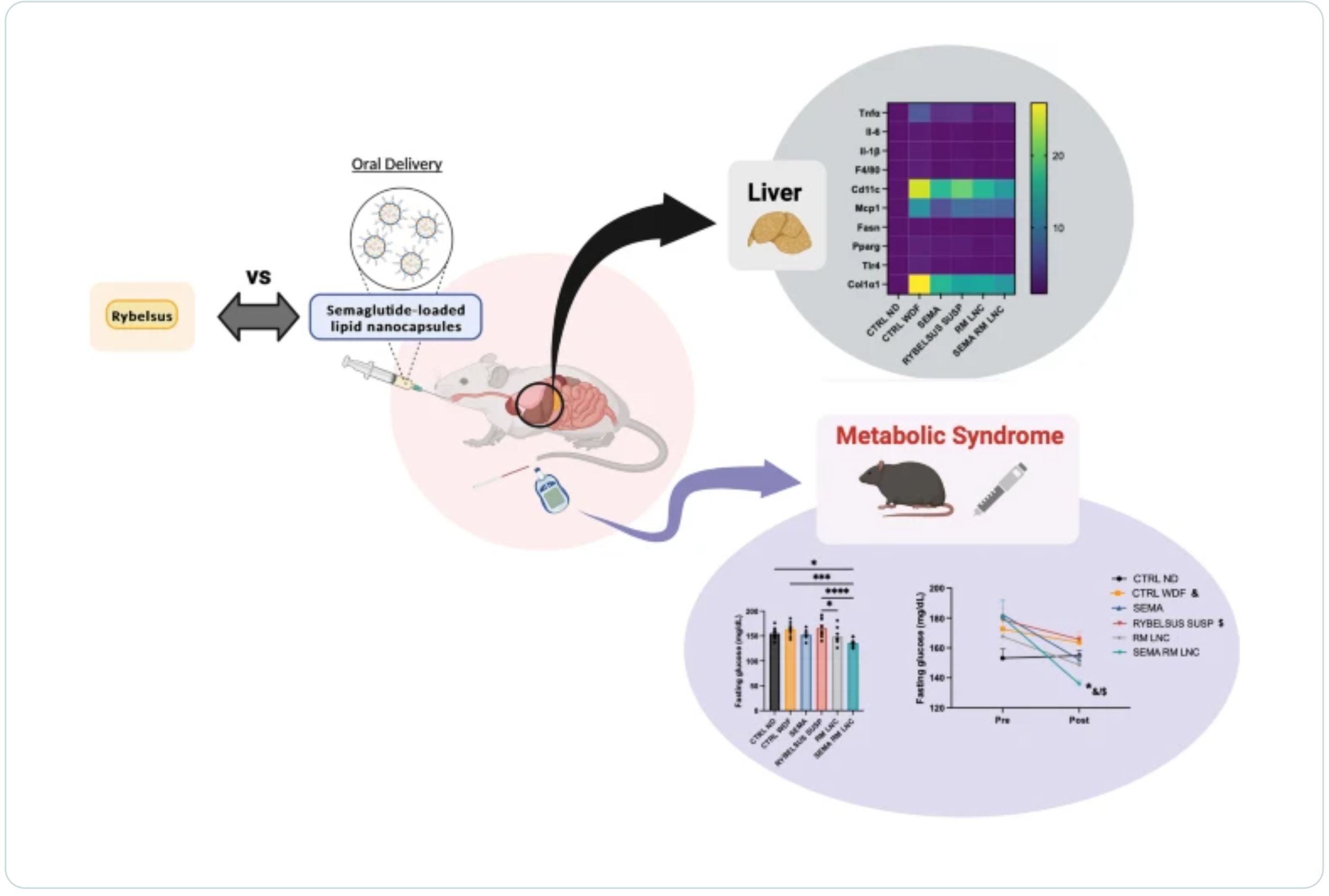
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a highly prevalent chronic liver disease that can progress to end-stage conditions with life-threatening complications, but no pharmacologic therapy has been approved. Drug delivery systems such as lipid nanocapsules (LNC) are very versatile platforms that are easy to produce and can induce the secretion of the native glucagon-like peptide 1 (GLP-1) when orally administered. GLP-1 analogs are currently being studied in clinical trials in the context of MASLD. Our nanosystem provides with increased levels of the native GLP-1 and increased plasmatic absorption of the encapsulated GLP-1 analog (semaglutide).
Our goal was to use our strategy to demonstrate a better outcome and a greater impact on the metabolic syndrome associated with MASLD and on liver disease progression with our strategy compared with the oral marketed version of semaglutide, Rybelsus®. Therefore, we studied the effect of our nanocarriers on a dietary mouse model of MASLD, the Western diet model, during a daily chronic treatment of 4 weeks. Overall, the results showed a positive impact of semaglutide-loaded lipid nanocapsules towards the normalization of glucose homeostasis and insulin resistance.
In the liver, there were no significant changes in lipid accumulation, but an improvement in markers related to inflammation was observed. Overall, our strategy had a positive trend on the metabolic syndrome and at reducing inflammation, mitigating the progression of the disease. Oral administration of the nanosystem was more efficient at preventing the progression of the disease to more severe states when compared to the administration of Rybelsus®, as a suspension.
Introduction
Recently, a consensus on a new nomenclature has been proposed, with metabolic dysfunction-associated steatotic liver disease (MASLD) replacing nonalcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated steatohepatitis (MASH) replacing nonalcoholic steatohepatitis (NASH). This change was implemented due to problems with accurately capturing the etiology of the disease and in the use of stigmatizing language (“nonalcoholic” and “fatty”) [1]. In addition, the new definition includes at least one of five cardiometabolic risk factors, which are related to body mass index (BMI), fasting serum glucose, blood pressure, plasma triglycerides and plasma HDL-cholesterol levels, as diagnostic criteria [1, 2].
MASLD is a slow progressing chronic liver disease that results from a complex interplay of factors (environmental, genetic, lifestyle, etc.) [2]. These factors contribute to the gradual development of this highly prevalent metabolic liver disorder. The initial stage is known as metabolic dysfunction-associated steatotic liver (MASL), characterized by more than 5% of hepatocytes containing lipid droplets. Furthermore, MASH involves hepatic steatosis, lobular inflammation, hepatocyte injury with ballooning, and varying degrees of fibrosis. Advanced cases may lead to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [2]. In a significant number of patients, MASLD coexists with other dysmetabolic traits, such as obesity and type 2 diabetes mellitus (T2DM), suggesting that MASLD is the hepatic manifestation of the metabolic syndrome (MetS), impacting approximately 30% of the global adult population [2, 3]. Existing treatment strategies primarily rely on lifestyle modifications, such as exercise and dietary restrictions, as the sole validated therapeutic intervention. Despite demonstrating efficacy in reversing MASH, maintaining life-style changes over the long term is challenging for most patients, and there are currently no pharmacological treatments approved [4].
Incretin-like hormones, such as glucagon-like peptide-1 (GLP-1), exert their effects by inducing glucose-dependent effects on insulin secretion, inhibiting glucagon release, slowing gastric emptying, and reducing food intake, ultimately leading to weight loss [5, 6]. The rapid inactivation of GLP-1 by the enzyme dipeptidyl peptidase IV (DPP-IV) within minutes prompted the development of GLP-1 analogs with prolonged half-lives, which are now approved for the treatment of T2DM and obesity (semaglutide, 160 h half-life). GLP-1 analogs indirectly impact hepatic metabolism via its direct effects on the pancreas and central nervous system. This actions leads to reduced hepatic steatosis, inflammation, and fibrosis, though to a lesser extent [6]. When administered via subcutaneous injection, GLP-1 analogs have been extensively investigated in clinical trials for MASLD treatment and show promise as mono- or combination therapies. Examples include liraglutide (LEAN Project: ClinicalTrials.gov, number NCT01237119) [7] and semaglutide (ClinicalTrials.gov, number NCT02970942) [8], both of which have completed phase II trials, meeting their primary endpoint of NASH resolution without worsening fibrosis. A phase III clinical trial for semaglutide has already been initiated (ClinicalTrials.gov, number NCT04822181). Moreover, Rybelsus®, an oral formulation of semaglutide, is currently being tested in 2 ongoing studies (ClinicalTrials.gov, numbers NCT05813249 and NCT03919929).
In previous research from our group, we demonstrated that oral drug delivery systems, such as lipid nanocapsules (LNC), effectively induce the release of endogenous GLP-1 [9]. Exenatide (EXE, half-life 2.5 h), an exogenous GLP-1 analog, has been successfully incorporated into these nanocarriers. This dual-action strategy (increased secretion of the native GLP-1 and increased absorption of a GLP-1 analog) has shown to be effective in a mouse model of T2DM. This approach not only impacted glucose homeostasis but also produced positive effects on hepatic steatosis, surpassing the outcomes observed with the subcutaneous injection of exenatide [9]. The oral administration of incretin mimetic peptides has the additional therapeutic advantage of simulating the normal physiological pathway of the native peptide. Orally administered GLP-1 analogs can access the liver at much higher concentrations via the hepatic portal vein than via subcutaneous delivery, reducing systemic exposure.
Therefore, we first selected exenatide to test the therapeutic effect of tour strategy on MASLD. We hypothesized that the increase in endogenous GLP-1 levels induced by lipid nanocapsules could reach therapeutic levels in the context of MASLD. In a previous MASLD study, we observed that exenatide-loaded lipid nanocapsules and blank nanoparticles (RM-LNC) had similar effects [10], which prompted the exploration of a more potent GLP-1 analog—semaglutide (SEMA). Therefore, we tested the effects of the blank and semaglutide-loaded lipid nanocapsules (SEMA-RM-LNC) on metabolic and liver parameters, in a rodent model of MASLD, and compared them with those of Rybelsus®, the oral marketed version of semaglutide that is available in the form of tablets and administered to the mice as a suspension.
The final goal of this study was to evaluate the impact of our nanosystem on the progression of the disease in a mouse model of early MASH (without fibrosis) when undergoing chronic treatment (one month) versus the oral administration of the peptide alone and its oral marketed version in a suspension form. Furthermore, we indirectly compared the results obtained with our previous studies using exenatide [10].
Download the full article as PDF here Effects of semaglutide-loaded lipid nanocapsules on metabolic dysfunction-associated steatotic liver disease
or read it here
Materials
Labrafac® WL 1349 (caprylic/capric acid triglycerides) and Peceol® (oleic acid mono-, di- and triglycerides) were obtained from Gattefossé (Saint-Priest, France). Lipoid® S100 (soybean lecithin at 94% of phosphatidylcholines) was obtained from Lipoid GmbH (Ludwigshafen, Germany). Kolliphor® HS15 (12-hydroxystearate PEG 660 and PEG 660), Span 80® (Sorbitan Oleate) and sodium chloride (NaCl) were purchased from Sigma-Aldrich (St. Louis, USA). Semaglutide was purchased from Bachem (Bubendorf, Switzerland). Dipeptidyl peptidase IV (DPP-IV) inhibitor was purchased from Millipore (St. Charles, USA). Three mg Rybelsus® tablets were purchased from a community pharmacy in Brussels. All chemical reagents used in this study were of analytical grade.
Domingues, I., Yagoubi, H., Zhang, W. et al. Effects of semaglutide-loaded lipid nanocapsules on metabolic dysfunction-associated steatotic liver disease. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01576-z
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


