Evolution of the Microstructure of Sustained-release Matrix Tablets during Dissolution and Storage
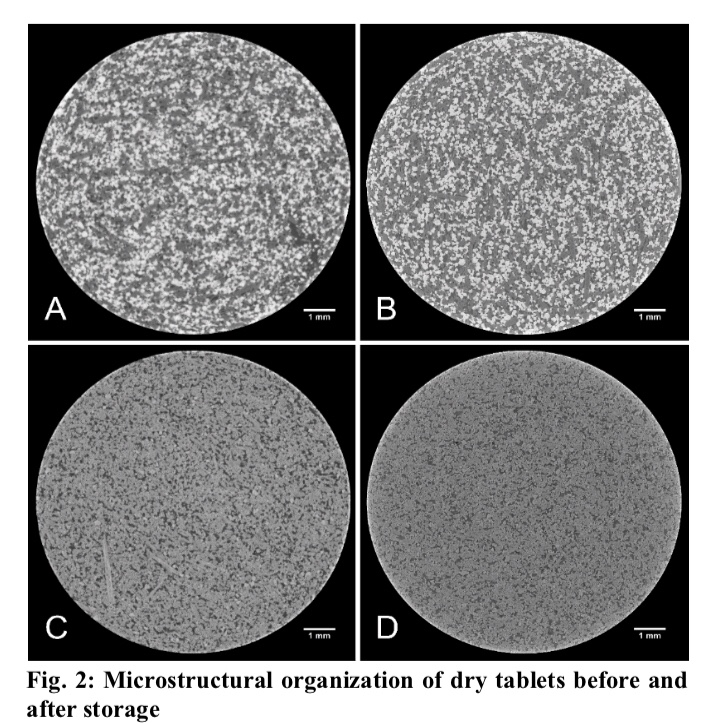
Glyceryl behenate based-matrix tablets allowed obtaining a sustained release of theophylline for up to 12 hours. The diluent selected as pore-forming agent (lactose or dibasic calcium phosphate anhydrous) affected the drug release and its stability after 3-month storage at 40°. Lactose-containing matrix tablets exhibited a quicker drug release than those containing dibasic calcium phosphate anhydrous because of its higher water-solubility. After 3-month storage at 40°, tablets containing lactose showed the same drug release whereas a significantly slower release was observed for dibasic calcium phosphate anhydrouscontaining tablets. Synchrotron radiation-based X-ray micro computed tomography was used for the evaluation of eventual changes in the microstructure of the matrix tablets during dissolution and after longterm storage. X-ray images provided new insights into the interplay of pharmaceutical ingredients and the importance of excipient surface structure on drug release overtime. The solid-lipid excipient could be adsorbed on the textured surface of mesoporous dibasic calcium phosphate anhydrous resulting in reduced water permeability of the matrix and therefore slower drug release. These findings provide formulators with fundamental formulation considerations for the development of sustained-release matrix tablets using glyceryl dibehenate as release retarding agent.
Download full article here: evolution-of-the-microstructure-of-sustainedrelease-matrix-tablets-during-dissolution-and-storage-3.pdf

