In vitro lipolysis test in lipid-based formulation development

The lipolysis test is recommended during lipid-based formulation (LBF) development to evaluate whether the prototype formulations should be able to solubilize and maintain the drug in solution during the ‘in vivo’ digestion process. It is also a good tool to discriminate between formulations and select the best formulation for further development.
When use the lipolysis test?
Lipid-based formulations enable the solubilization of poorly water soluble drugs (BCS class 2 and 4). They are usually developed stepwise:
- Assess solubility of the drug in individual excipients (oils, surfactants and solvents) and select the excipients with highest solubilization capacity.
- Perform miscibility, dispersion testing and ternary phase diagramming to select the best excipient combination and define their ratios to develop the LBF prototypes.
- Perform the in vitrolipolysis test of the prototypes to assess if the drug is maintained in a solubilized state throughout the digestion process and select the best prototype for further development and studies.
The detailed protocol is described in Gattefossé formulation guideline entitled “Lipid Based Formulations for Oral Bioavailability Enhancement” available upon request. This systematic approach is straightforward and enables successful and fast LBF development (Dave and Le Pree, 2017).
Theoretical background
The lipolysis testing was standardized and validated by the Lipid Formulation Classification Consortium (Williams H.D. et al, 2012a; Williams H.D. et al, 2012b; Sassene, P.J et al, 2014; Bakala-N’Goma et al, 2015).
The objective of the lipolysis test is to assess the drug solubility in conditions that mimic the fasted intestinal tract in term of volume, pH, temperature and enzymes. It helps define the extent of digestion by measuring the amount of fatty acids released in the lipolysis medium. The test helps determine the solubility of the drug during a simulated digestion process.
Practical description of the test
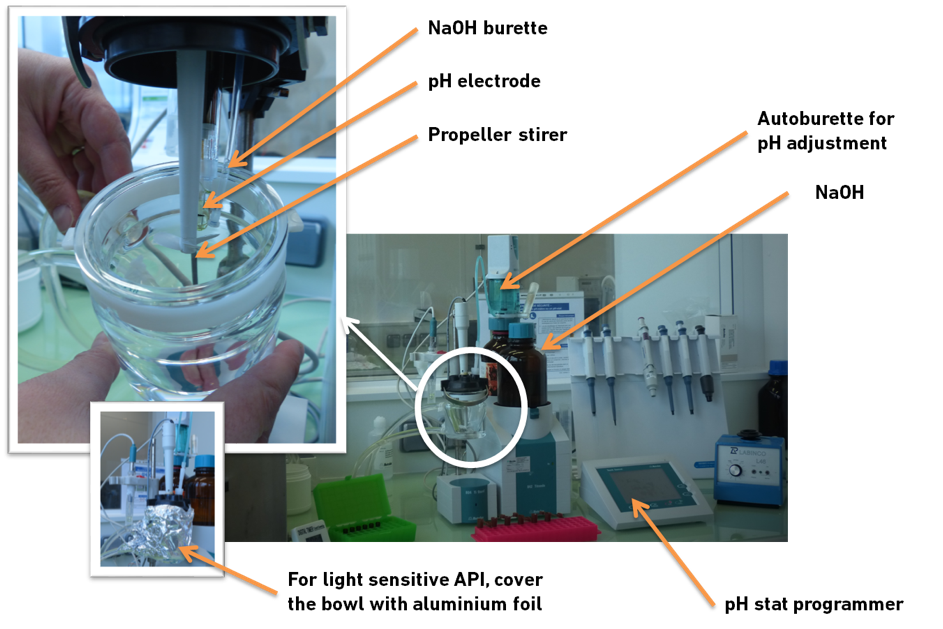
Material
The assay is performed with a pH-stat apparatus filled with lipolysis medium, to mimic fasted state in the small intestine and with the temperature set to 37°C.
A two-step method
To evaluate the impact of digestion on the formulation and its ability to maintain the drug in solution, the formulation is dispersed in aqueous medium for 10 minutes followed by addition of lipases. Aliquots of the dispersion are taken at different intervals for subsequent testing.
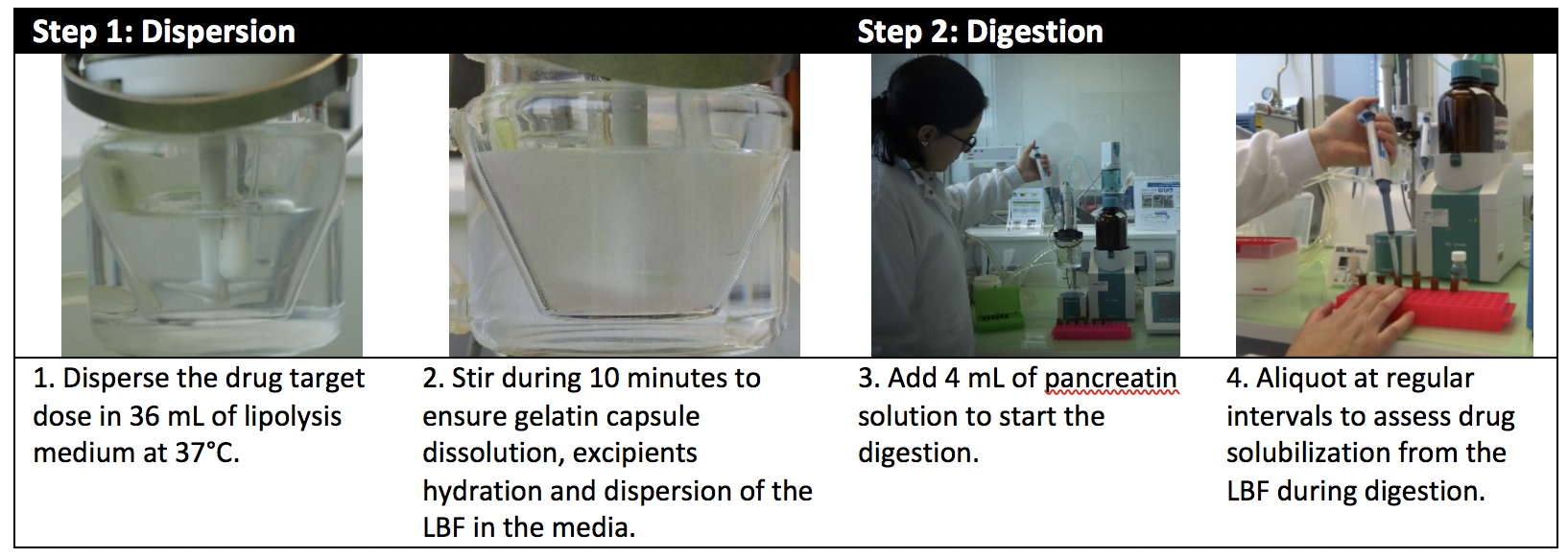
As soon as aliquots are taken, an enzyme inhibitor is added to stop the reaction. The sample is then centrifuged at 37°C. The drug is dosed in each phase: oily phase (non-dispersed), aqueous phase (dissolved in the micelles) and pellet phase (precipitated drug). The quantity of drug solubilized in aqueous phase is plotted versus time.
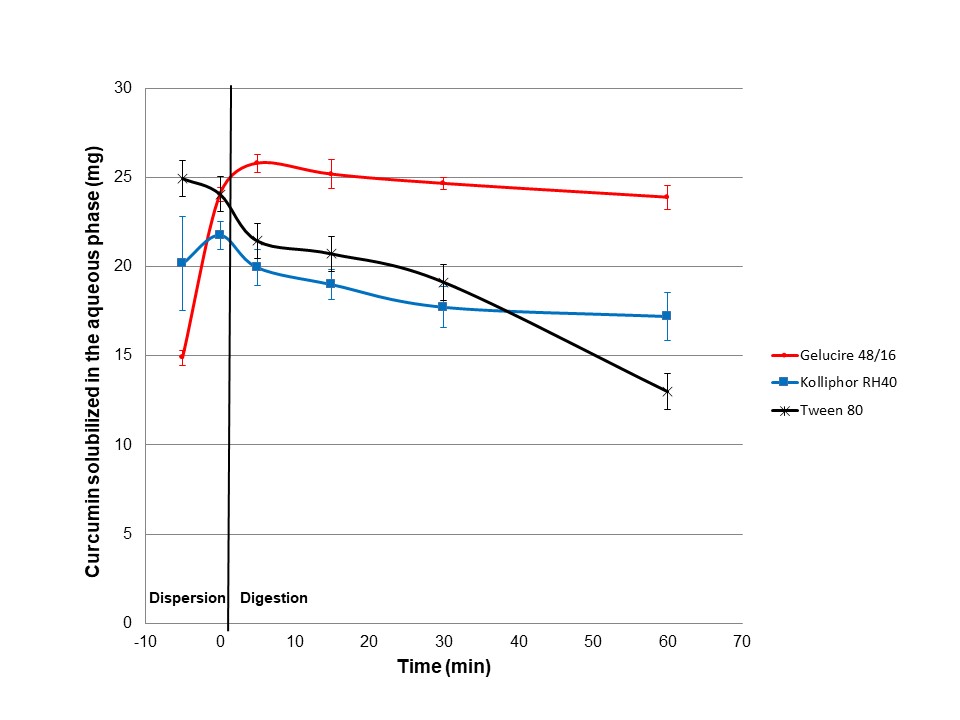
Case study with curcumin
Jannin et al, 2015 used the lipolysis test to discriminate between different formulations. They showed that the formulation containing Gelucire®48/16 as surfactant enables the solubilization of the target drug dose, throughout the digestion process, whereas other formulations showed high propensity to precipitate. Detailed formulations are given in the paper as well as case studies with piroxicam and nifedipine.
![]()
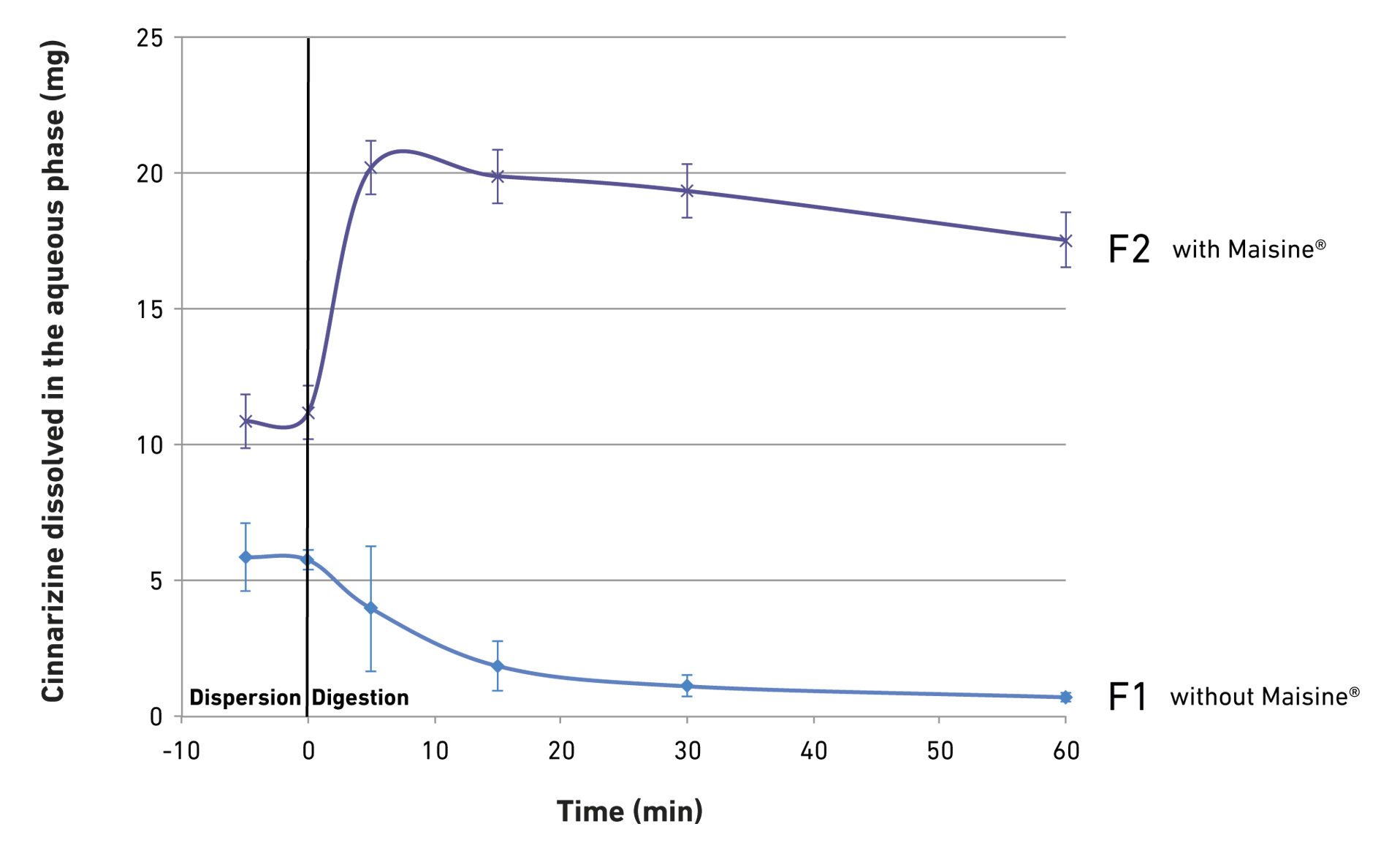
Case study with cinnarizine
Solubility screening of drug with lipid-based excipients led to the development of an initial LBF containing Labrasol® ALF as water dispersible surfactant and Capryol™ 90 as water insoluble surfactant. This initial formulation was able to solubilize the entire dose when dispersed into 250 ml of water at 37°C (formulation F1). However, using the lipolysis assay, the formulation was shown to be unable to maintain drug in a solubilized state during digestion. The formulation was optimized by the addition of Maisine® CC to enhance drug solubility through micellization (F2).
Conclusions
The in vitrolipolysis test is a discriminative and predictive tool to assess if the formulation will solubilize the target dose and maintain the drug in solution during the digestion. It also provides indications on how formulations could be improved.
More information at https://www.gattefosse.com/in-vitro-lipolysis-test
References
Bakala-N’Gomaet al. (2015) Toward the Establishment of Standardized in vitro Tests for Lipid-Based Formulations. Part 5. Lipolysis of representative formulations by gastric lipase. Pharm. Res., 32 (4) 1279-1287
Dave M. and Le Pree J.(2017) A Systematic Approach to Lipid-Based Formulation Development for a Poorly Soluble API, Fenofibrate. AAPS poster
Jannin V. et al (2015). Development of self emulsifying lipid formulations of BCS class II drugs with low to medium lipophilicity. International Journal of Pharmaceutics, Volume 495, Issue 1, 10 November 2015, Pages 385-392
Sassene et al. (2014) Toward the Establishment of Standardized In vitro Tests for Lipid-Based Formulations, Part 6: Effects of Varying Pancreatin and Calcium Levels. AAPS J., 16 (6) 1344-1357
Williams H.D et al. (2012a) Toward the establishment of standardized in vitro tests for lipid-based formulations. Part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J.Pharm. Sci. 101: 3360-3380.
Williams H.D. et al. (2012b) Toward the establishment of standardized in vitro tests for lipid-based formulations, Part 2: The effect of bile salt concentration and drug saturation level (dose) on the performance of Type I, II, IIIA, IIIB and IV formulations during in vitro digestion. Mol. Pharmaceut. 9: 3286 -3300.