Fabrication of a Controlled-Release Core-Shell Floating Tablet of Ketamine Hydrochloride Using a 3D Printing Technique for Management of Refractory Depressions and Chronic Pain
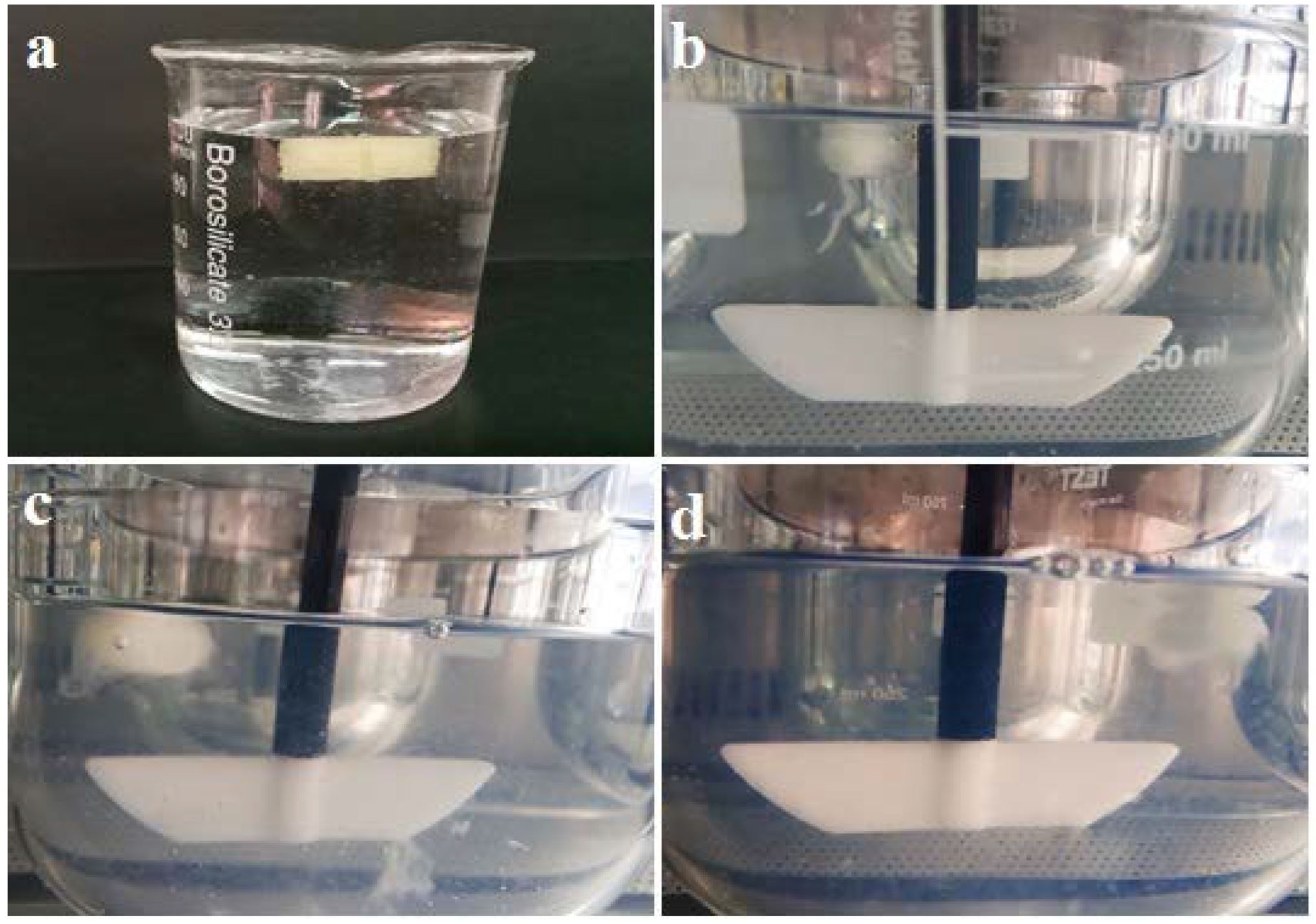
In this study, a novel floating, controlled-release and core-shell oral tablet of ketamine hydrochloride (HCl) was produced using a dual extrusion by 3D printing method. A mixture of Soluplus® and Eudragit® RS-PO was extruded by a hot-melt extrusion (HME) nozzle at 150–160 °C to fabricate the tablet shell, while a second nozzle known as a pressure-assisted syringe (PAS) extruded the etamine HCl in carboxymethyl cellulose gel at room temperature (25 °C) inside the shell. The resulting tablets were optimized based on the United States pharmacopeia standards (USP) for solid dosage forms.
Moreover, the tablet was characterized using Fourier-transform infrared (FTIR) spectrum, scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and buoyancy techniques. The results showed a desired dissolution profile for a 100% infill optimized tablet with total drug release (100%) during 12 h. Weight variation and content uniformity of the tablets achieved the USP requirements. SEM micrographs showed a smooth surface with acceptable layer diameters. According to the FTIR analysis, no interference was detected among peaks. Based on DSC analysis, the crystallinity of ketamine HCl did not change during melt extrusion.
In conclusion, the floating controlled-release 3D-printed tablet of ketamine HCl can be a promising candidate for management of refractory depressions and chronic pain. Additionally, the additive manufacturing method enables the production of patient-tailored dosage with tunable-release kinetics for personalized medicine in point-of care setting.
Download the full article as PDF here Fabrication of a Controlled-Release Core-Shell Floating Tablet of Ketamine Hydrochloride Using a 3D Printing Technique for Management of Refractory Depressions and Chronic Pain
or read it here
Materials
Ketamine hydrochloride solution (CAS: 1867-66-9) was purchased from Delphis pharmaceutical (Gujarat, Rajkot, India) and was freeze-dried to create a powder form with a purity of approximately 98.7%. Soluplus® (CAS: 402932-23-4), Eudragit® RS-PO (CAS: 33434-24-1), and carboxymethyl cellulose (CMC) (CAS: 9004-32-4) were provided from BASF (Ludwigshafen, Germany), Degusa-huels Gruppe (Frankfurt, Germany), and Sigma-Aldrich (Darmstadt, Germany) companies, respectively. Ethanol solvent (CAS: 64-17-5, Purity ≥ 98%) was purchased from Razi manufacturing, Tehran, Iran.
Karami, T.; Ghobadi, E.; Akrami, M.; Haririan, I. Fabrication of a Controlled-Release Core-Shell Floating Tablet of Ketamine Hydrochloride Using a 3D Printing Technique for Management of Refractory Depressions and Chronic Pain. Polymers 2024, 16, 746. https://doi.org/10.3390/polym16060746
Read also our introduction article on 3D Printing here:


