Investigating the Influence of HPMC K4M and Eudragit L 100-55 on Guanfacine-Loaded Extended-Release Tablets
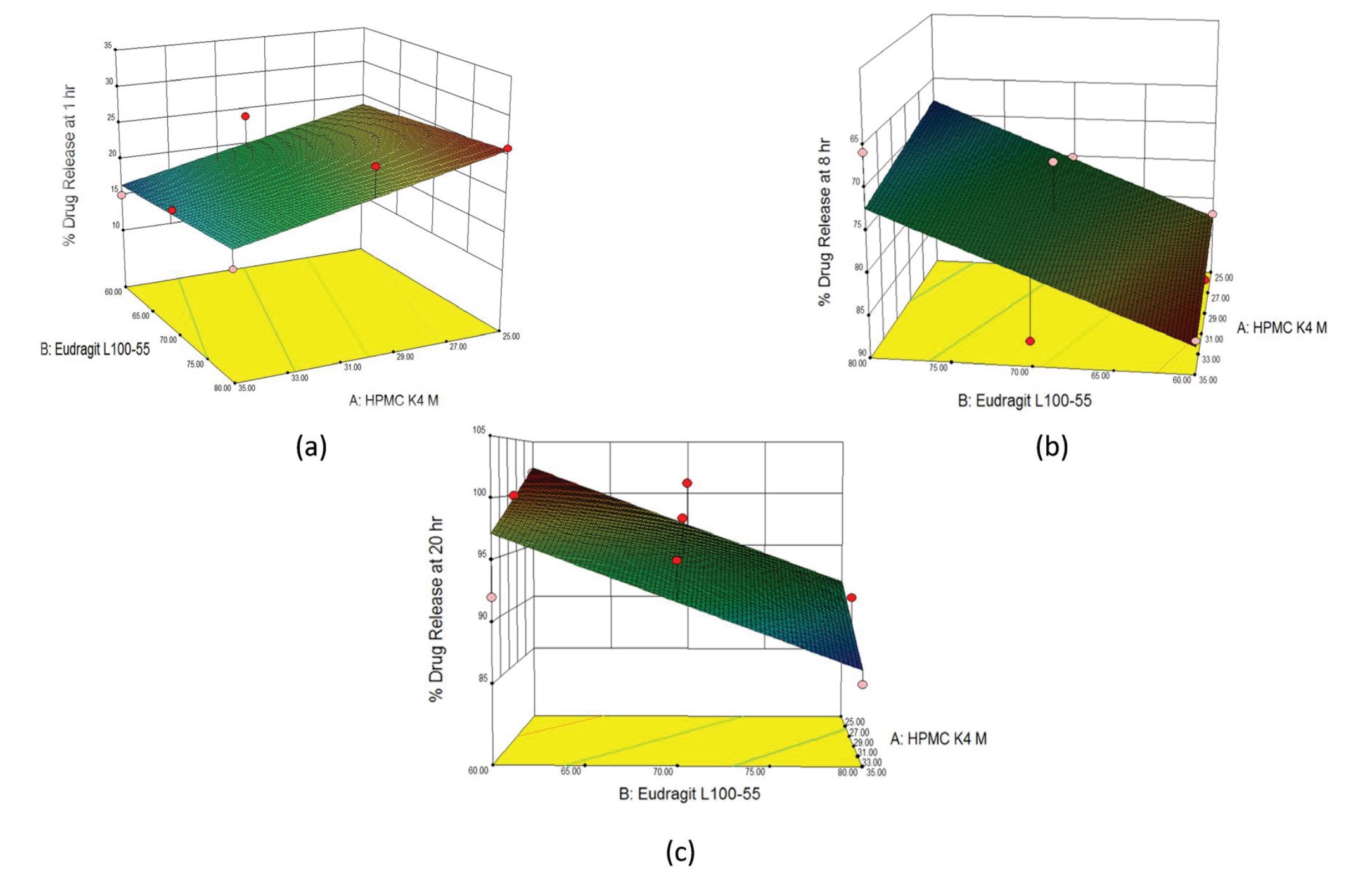
This study aims to optimize the concentrations of hydroxypropyl methyl cellulose (HPMC) K4M and Eudragit L100- 55 (methacrylic acid) in formulation of extended-release tablets containing guanfacine hydrochloride by employing a 32 -factorial design approach. Extended-release tablets of guanfacine hydrochloride reduce the need for frequent dosing to achieve the desired therapeutic outcomes. Cumulative drug release after 1, 8, and 20 hours of dissolution were taken as target responses and concentrations for both polymers as the variables.
3D response surface and polynomial equations were generated for choosing the optimized formulation with the most favorable response. Suitability of the drug and excipients was assessed during preliminary evaluation and compatibility studies using an accelerated thermal stress study. The release kinetics of the formulations followed Hixson Crowell and Higuchi models, indicating slow erosion of polymer to release the drug over an extended time period. Validated optimization techniques confirmed predictability of the model. The stability study verified superiority of the optimized formulation after 3 months of storage.
Download the full article as PDF here Investigating the Influence of HPMC K4M and Eudragit L 100-55 on Guanfacine-Loaded Extended-Release Tablets
or read it here
Materials
Guanfacine hydrochloride was obtained from Intas Pharmaceuticals Ltd.(Ahmedabad, Gujarat). HPMC K4M (molecular weight: 1261.4 g/mol, degree of substitution: 20–24% of methoxyl and 7–12% of hydroxypropyl substitutions) was procured from Samsung Fine Chemicals Co., Ltd (Korea). Eudragit L100-55 (methacrylic acid) was purchased from Evonik Industries Signet Chemical Corporation Pvt. Ltd (Maharashtra, India). Microcrystalline cellulose PH-102 was from FMC Asia – Pacific, Inc. (Maharashtra, India). Isopropyl alcohol was obtained from Rankem (Gujarat, India). Lactose monohydrate was obtained from Tiwari Chemicals and Tiwari Pharma (Himachal Pradesh, India). Citric acid and fumaric acid were obtained from Thirumalai Chemicals (Maharashtra, India). Glyceryl behenate was obtained from Gattefosse, Ltd. (India). Lake of Indigo carmine and ferric oxide yellow were from Colorcon (West Point, PA, USA). All chemicals and reagents used were of analytical grade.
Rakesh P. Patel, Himanshu Paliwal and Aesha Patel, Investigating the Influence of HPMC K4M and Eudragit L 100-55 on Guanfacine-Loaded Extended-Release Tablets, Department of Pharmaceutics and Pharmaceutical Technology, Shree S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Mehsana, India. dx.doi.org/10.14227/DT300123P22

