Semisolid Wet Sol–Gel Silica/Hydroxypropyl Methyl Cellulose Formulation for Slow Release of Serpin B3 Promotes Wound Healing In Vivo
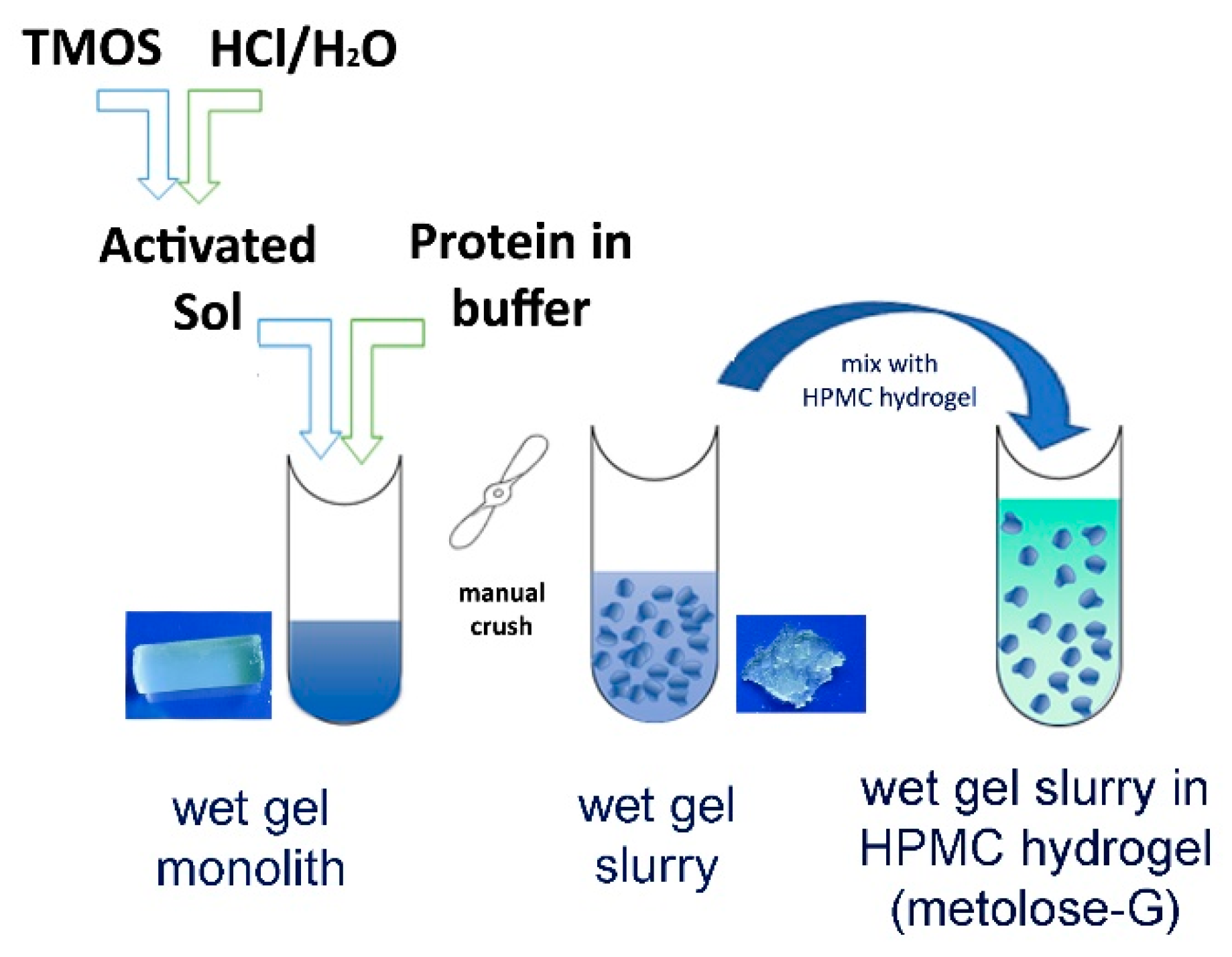
Foot ulcerations are a disabling complication of diabetes and no treatment is currently available based on disease mechanisms. The protein serpin B3 (SB3) was identified as a positive biomarker of successful diabetic wound healing; therefore, its exogenous administration may promote healing. The topical administration of SB3 is challenging due to its protein nature. Physical entrapment in wet sol–gel silica can stabilize the protein’s conformation and permit its sustained delivery. However, irreversible syneresis and poor viscoelastic properties hamper wet sol–gel silica application as a semisolid vehicle. To overcome these limits, a sol–gel silica/hydroxypropylmethylcellulose (HPMC) hydrogel blend was developed. SB3 entrapped in 8% SiO2 wet sol–gel silica preserved its structure, was stabilized against denaturation, and was slowly released for at least three days. Blending a silica gel with an HPMC–glycerol (metolose-G) hydrogel permitted spreadability without affecting the protein’s release kinetics. When administered in vivo, SB3 in silica/metolose-G—but not in solution or in metolose-G alone—accelerated wound healing in SB3 knockout and diabetic mouse models. The results confirmed that SB3 is a new pharmacological option for the treatment of chronic ulcers, especially when formulated in a slow-releasing vehicle. Silica–metolose-G represents a novel type of semisolid dosage form which could also be applied for the formulation of other bioactive proteins.
Materials and Instrumentation
BSA–FITC was obtained through the conjugation of BSA with 10 equivalents of FITC in PBS. The product (FITC:BSA = 2:1 mol:mol) was purified by extensive dialysis (10 kDa cut-off) against PBS, sterile filtered, and maintained at 4 °C until use. Spectroscopic analyses were performed with a Varian Cary 50 UV-Vis spectrophotometer. The fluorescence was measured using a JASCO FP-6200 spectrofluorometer. Circular dichroism spectra were recorded using a JASCO J-810 spectropolarimeter. Protein release studies were conducted using 1 mL Float-A-Lyzer G2 dialysis tubes from Spectrum Laboratories, Inc. A Hettich Universal 320-R centrifuge was used for the gel washes. A Timo autoclave was used for sterilization. A Memmert GmbH, model SV 1422, was used as a thermostated bath. Electrophoresis on a polyacrylamide gel was conducted using a gel casting from the Bio-Rad Mini-Protean System. Western blots were performed on Millipore PVDF (polyvinylidene fluoride) membranes. The Molecular Imager VersaDoc MP4000 was used for the acquisitions of the Western blot membranes.
Download the full study as PDF here Semisolid Wet Sol–Gel Silica/Hydroxypropyl Methyl Cellulose Formulation for Slow Release of Serpin B3 Promotes Wound Healing In Vivo
or read it here
Albiero, M.; Fullin, A.; Villano, G.; Biasiolo, A.; Quarta, S.; Bernardotto, S.; Turato, C.; Ruvoletto, M.; Fadini, G.P.; Pontisso, P.; Morpurgo, M. Semisolid Wet Sol–Gel Silica/Hydroxypropyl Methyl Cellulose Formulation for Slow Release of Serpin B3 Promotes Wound Healing In Vivo. Pharmaceutics 2022, 14, 1944. https://doi.org/10.3390/pharmaceutics14091944

