Improving API Solubility – by Merck
Overview of the article
- The Importance of API Solubility for Solid and Liquid Dosage Forms
- The Solubilization Process
- The Biopharmaceutics Classification System of APIs
- The Developability Classification System
- The Central Role of Solubility and Permeability for API Absorption
- Strategies for Enhancing API Solubility
- A Path for Addressing API Solubility Challenges
- The Value of a Trusted Partner
THE IMPORTANCE OF API SOLUBILITY FOR SOLID AND LIQUID DOSAGE FORMS
A sufficient level of solubility is critical to the efficacy of small molecule drugs – whether in a solid or liquid dosage form.1 In oral administration, the active pharmaceutical ingredient (API) must be absorbed by the body to become bioavailable and enter the systemic circulation to deliver the intended physiological effect. Poor solubility on the other hand results in low absorption from the gastrointestinal (GI) tract, with the API potentially not reaching therapeutic levels at the site of action. For parenteral formulations, good API solubility is critical as well as a prerequisite for particle free solutions and can be even more challenging in the case of high concentrated formulations. If an API that is orally administered via a solid dosage form does not fully or just partly dissolves within GI fluids, it cannot permeate through the intestinal membrane and does not achieve the desired therapeutic effect. Many APIs that are currently on the market suffer from poor solubility. However, this challenge will become increasingly important as up to 90% of all drugs in the small molecule development pipeline are estimated to be poorly soluble. If this problem cannot be addressed, a promising medication may never make it to the market.2
THE SOLUBILIZATION PROCESS
High lipophilicity and strong intermolecular forces impact solubilization of drug molecules.1 Solubility is the ability of a solute to dissolve in a solvent, forming a homogenous mixture. The process of solvation can be broken down into three steps as diagrammed in Figure 1.
- A solute molecule must be separated from the solid state by breaking solute-solute bonds.
- A gap must be created within the solvent.
- The solute molecule must be incorporated within the solvent.
Equilibrium solubility is defined as the point at which the dissolution rate is equal to the precipitation rate and is the maximum amount of substance that can be dissolved in a stable equilibrium.

The measure of equilibrium is a thermodynamic value and does not take into account the rate at which the system reaches equilibrium. Because of this, such measurements can be misleading, If a compound has acceptable equilibrium solubility but also a poor dissolution rate, the compound may not reach the expected concentration levels in the GI tract before being cleared out of the body. In this situation, absorption may not reach the desired level.
Figure 2 shows the process of dissolution. For a drug particle to dissolve, a concentration gradient must be present between the drug in free solution (C) and the drug concentration on the surface of the dissolving solid (Cs), separated by a layer of unstirred solvent (h).
This gradient allows diffusion of the solid that is in the process of dissolving through the unstirred layer and into solution. The rate of dissolution is thus equal to the rate of diffusion through the unstirred solvent layer.

THE BIOPHARMACEUTICS CLASSIFICATION SYSTEM OF APIS
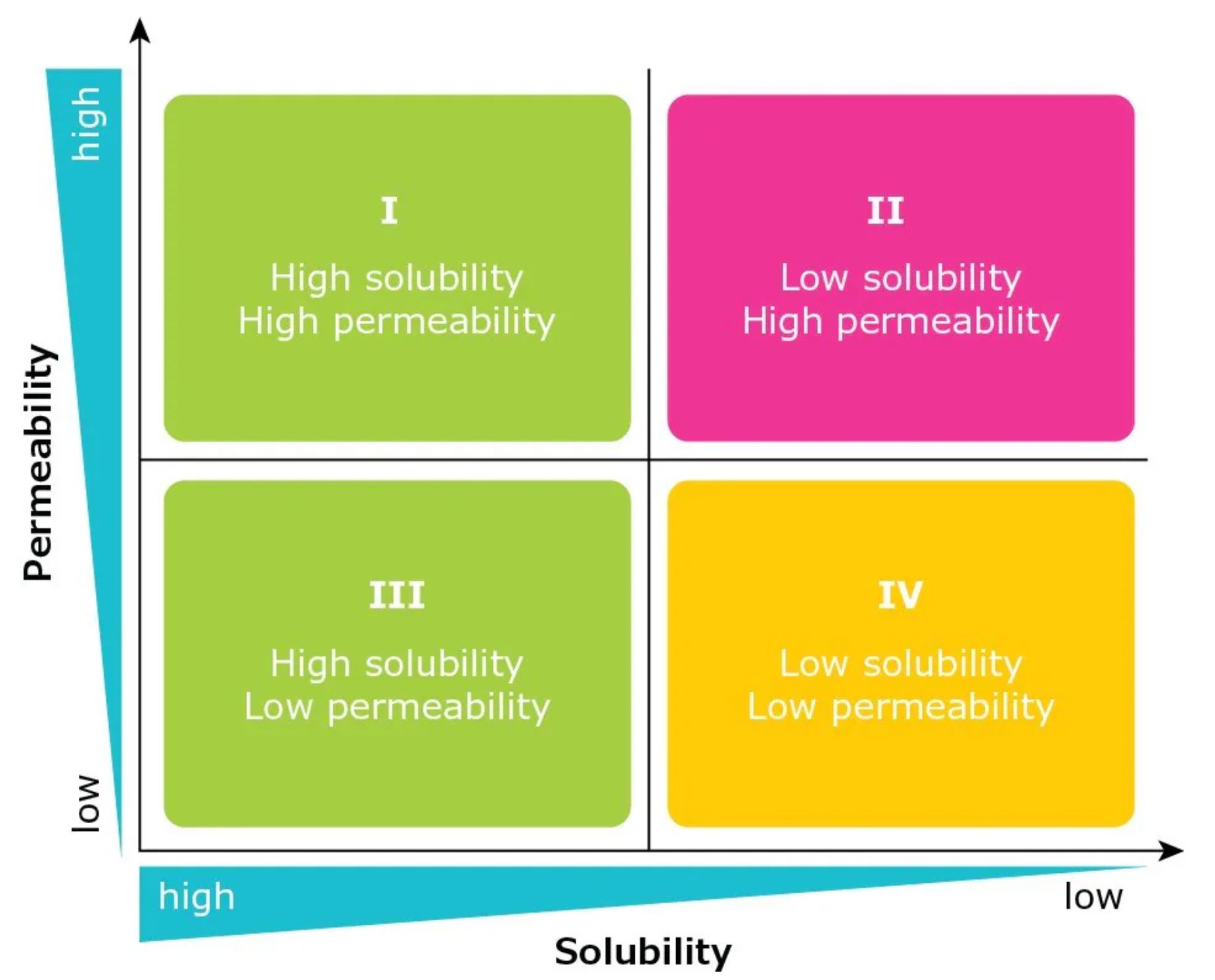
The importance of API solubility is reflected in the Biopharmaceutics Classification System (BCS). Developed in the 1990s, the BCS categorizes APIs in terms of their solubility and permeability and correlates this with their potential in vivo performance (Figure 3).3 The system was originally used by the United States Food and Drug Administration (FDA) as the basis for biowaiver applications.
As defined by the BCS, Class I molecules exhibit good solubility and good permeability, and are expected to have good absorption in the GI tract. BCS Class II compounds have low solubility and high permeability, while BCS Class III molecules have high solubility and low permeability. The most challenging class of molecules have low solubility and low permeability and are categorized as BCS Class IV. Approximately 30-40% of all marketed drugs have low solubility (BCS Class II and IV).2
As high throughput screening and target-oriented drug discovery often result in poorly water-soluble APIs, the percentage of drugs and drug substances with this shortcoming is expected to increase. With an estimated 70–90% of drugs currently under development being poorly soluble, new strategies are needed to overcome this challenge (Figure 4).2
THE DEVELOPABILITY CLASSIFICATION SYSTEM
The BCS served as a basis for the Developability Classification System (DCS).4 The DCS added a solubility-limited absorbable dose (SLAD) line to further differentiate APIs within Class II (Figure 4). Drugs above the SLAD line are dissolution rate limited (DCS Class IIa), while those below are solubility limited (DCS Class IIb). Solubility is tested using biorelevant FASSIF (fasted state simulated intestinal fluid) media, providing a more accurate reflection of what is taking place in the human body. This allows formulation scientists to be less conservative and to shift the dose solubility ratio from 250 to 500.
Overall, the DCS allows formulation scientists to understand the root causes of a solubility challenge and distinguish between the two classes, providing important insights to guide efforts to address the problem.

The DCS categorizes APIs based on the factor responsible for poor absorption:
- Dissolution rate-limited: The dissolution rate is too slow for the API to dissolve during time of passage by the site of absorption (usually the small intestine).
- Solubility-limited: The solubility is too low for the required amount of API to dissolve in the available GI fluid volume.
- Permeability-limited: The rate of transfer from GI tract through the intestinal membrane is too low. There is limited influence on permeability by excipients choice and formulation.
Read the full article here
Source: Merck, Improving API Solubility (sigmaaldrich.com)

