Metamorphosis of Twin Screw Extruder-Based Granulation Technology: Applications Focusing on Its Impact on Conventional Granulation Technology
In order to be at pace with the market requirements of solid dosage forms and regulatory standards, a transformation towards systematic processing using continuous manufacturing (CM) and automated model-based control is being thought through for its fundamental advantages over conventional batch manufacturing. CM eliminates the key gaps through the integration of various processes while preserving quality attributes via the use of process analytical technology (PAT). The twin screw extruder (TSE) is one such equipment adopted by the pharmaceutical industry as a substitute for the traditional batch granulation process. Various types of granulation techniques using twin screw extrusion technology have been explored in the article. Furthermore, individual components of a TSE and their conjugation with PAT tools and the advancements and applications in the field of nutraceuticals and nanotechnology have also been discussed. Thus, the future of granulation lies on the shoulders of continuous TSE, where it can be coupled with computational mathematical studies to mitigate its complications.
INTRODUCTION
Pharmaceutical manufacturing has been carried out using batch processes since its inception, which, while tested and proved, are inept and financially exorbitant. These last several years, with the growing demand for solid dosage forms and expiring patents of drug products and molecules, acceleration, and de-risking of process and product development is of primal importance. Considering this, a change towards continuous manufacturing (CM) by the pharmaceutical industry and the regulatory bodies has drawn considerable attention by virtue of its inherent advantages towards process development, smaller physical footprint, manufacturing flexibility, product quality control, and overall expenditure to develop novel processes or by using familiar machinery in a non-traditional way (1,2,3). Continuous manufacturing (CM) is an up-and-coming technology in the pharma world that offers several benefits over the traditional batch processes, including production rate litheness, quality, robustness, and cost improvements (4, 5).
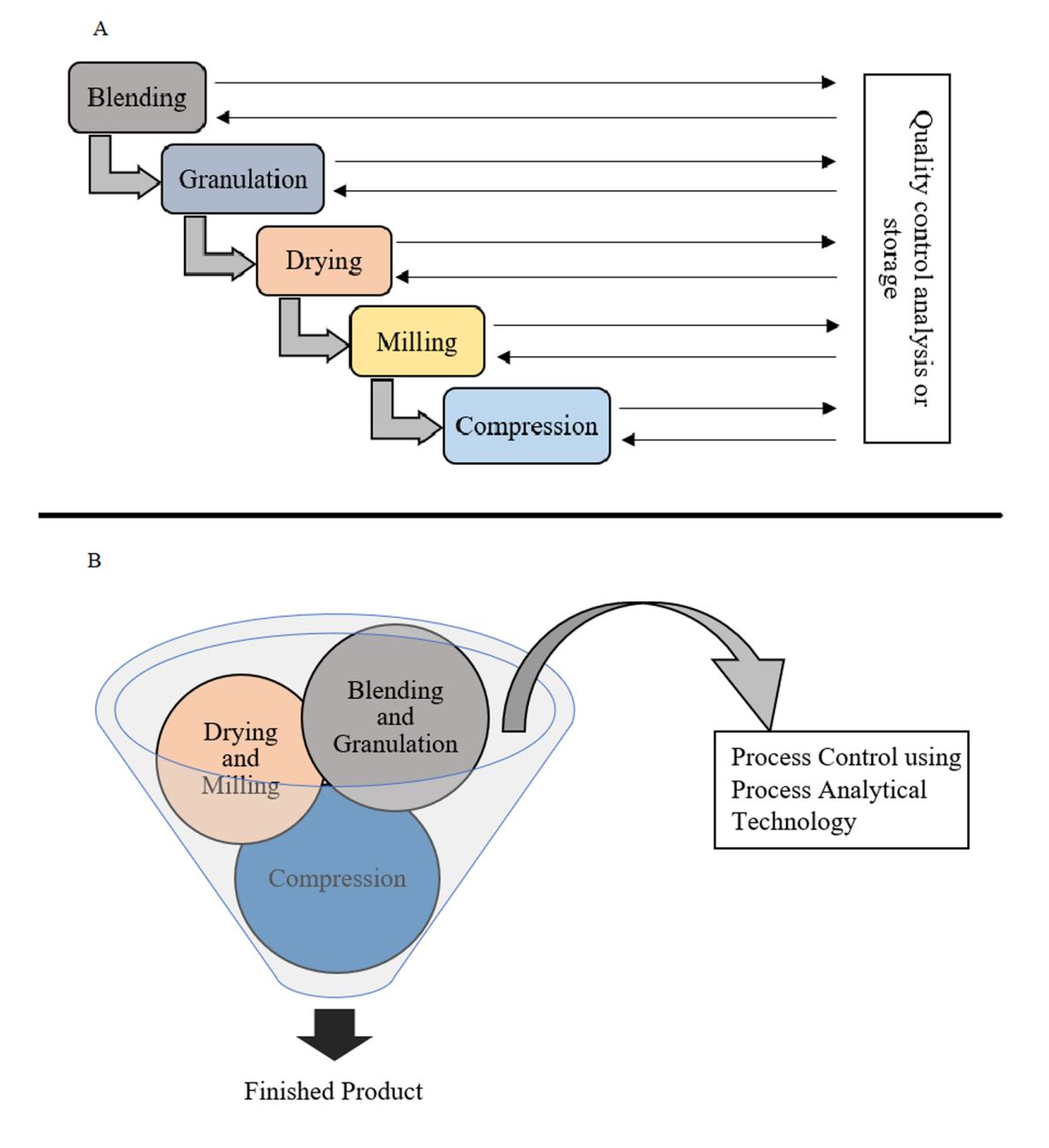
In a traditional solid drug product manufacturing process (Fig. 1A) that involves multiple processing steps (dispensing, mixing, high shear granulation, drying, milling, tableting, etc.), the FDA oversees each unit operation during manufacturing to ensure that the drug product supply is of high quality consistently. Previously, a lack of versatility and reproducibility caused many reservations in the pharmaceutical manufacturing industries, resulting in subpar drug products. This meant excessive additional work for the FDA so as to regulate practically every aspect of a certain manufacturing process (6). To keep up with the current market requirements and regulatory standards, most industries have initiated inculcating continuous manufacturing, a systematic approach to designing processes, and automated model-based control. This strategy has contributed to the immense quality and consistency improvements (7). A well-modeled continuous manufacturing system integrates multiple activities into a single production run (Fig. 1B) without compromising the quality by continuous tracking of critical process parameters (CPPs) and critical quality attributes (CQAs) via tests in the process flow using process analytical technology (PAT). This helps in the detection of variations in real time and corrects them to keep the process and the CQAs of the finished product within the standard limits. CM avoids the compulsion for managing process intermediates and helps with reducing the process cycle and finished product timeline (8, 9).
Download the full article as PDF here Metamorphosis of Twin Screw Extruder-Based Granulation Technology_Applications Focusing on Its Impact on Conventional Granulation Technology
or read it here
Rao, R.R., Pandey, A., Hegde, A.R. et al. Metamorphosis of Twin Screw Extruder-Based Granulation Technology: Applications Focusing on Its Impact on Conventional Granulation Technology. AAPS PharmSciTech 23, 24 (2022). https://doi.org/10.1208/s12249-021-02173-w

