Manufacturing pharmaceutical mini-tablets for pediatric patients using drop-on-demand printing
Abstract
The pharmaceutical industry has traditionally manufactured medicines in a limited range of dose strengths, with an emphasis on addressing the needs of the largest patient subgroups. This has disadvantaged smaller patient subsets, such as children, who often cannot find drug products in dosage levels suitable for their requirements. In recent years, development of pharmaceutical mini-tablets has emerged as an attractive solution to this problem. These are small-size dosages that offer attractive features such as flexible and personalized drug dosing, ease of swallowing, and tailored drug release, making them an excellent choice for administering medicines to children.
This study presents a novel technique for manufacturing pharmaceutical mini-tablets, using a drop-on-demand (DoD) printing system. In this method, a DoD system is used to generate precise droplets of a melt-based formulation, which are then captured and solidified in an inert solvent bath to produce individual mini-tablets. The study also evaluates the performance of this technique for various formulations, and quantifies two critical quality attributes (CQAs) of the resulting mini-tablets: content uniformity and dissolution behavior. The findings demonstrate that the manufactured mini-tablets can meet regulatory specifications for both CQAs, and that their release profiles can be customized by modifying the excipients used. The study also discusses promising areas of application and limitations of this technique.
Introduction
The advent of industrialization marked a fundamental shift in the way medicines are manufactured. Mass-produced dosages ushered in an era of safe, efficacious, and lower cost drugs, while simultaneously making them accessible to a broad section of the population. Mass-manufacturing of pharmaceuticals is, however, also challenged by a number of limitations, which have come under increased scrutiny in recent times. Lack of personalization, slow and inefficient supply chains, and drug shortages triggered by product recalls are some of the drawbacks that have adversely impacted therapeutic outcomes (Lee et al., 2015). Among these, the inability to produce personalized pharmaceutical products is a particularly pressing problem. Mass manufactured dosages often are made at a few discrete dose strengths that are suitable for the broad population. This ‘one-dose-fits-all’ approach leaves patient groups like pediatrics, geriatrics, and those with metabolic or organ dysfunction disadvantaged, as their drug dosing needs differ significantly from the populace (Trenfield et al., 2018). This study focuses on pediatric patients, by introducing a novel technique to manufacture pharmaceutical dosages catering to their requirements.
Pediatric patients span a broad age range, from newborn infants and toddlers to adolescents, and they differ from the adult population in several ways. Children show distinct pharmacokinetic and pharmacodynamic responses to drugs due to variations in metabolic rate, gastrointestinal absorption and renal function. This heightens the risk of drug and excipient related toxicity as their uptake and action may not follow expected adult pathways. Children also experience rapid development in physiological and cognitive functions in their early years. Thus the therapeutic dose of medicines required in this age group changes significantly, in turn necessitating highly flexible dosing. Other factors like ease of swallowing and taste masking are also crucial to promoting patient compliance. The combined effect of these additional requirements and a small market size (pediatric medicines constitute <10% of the overall pharmaceutical market) poses a major challenge for pharmaceutical companies to effectively serve this sector (Ivanovska et al., 2014, Milne and Bruss, 2008).
Traditionally, pediatric patients have been treated using dosage forms like crushed or split tablets, liquid oral drug products, and extemporaneously prepared dosages, as they can satisfy some of the aforementioned requirements. Crushed or split tablets (or powder formulations) are attractive as they can be dispensed in individualized dose amounts and can be blended in with food for good taste masking. However, this technique risks altering the intended dissolution behavior of the drug product and risks impacting dosing accuracy as the split subunits may have non-uniform drug amounts. Liquid oral dosages are one of the most popular treatment forms used in pediatric therapy. They are easy to administer and consume and can be readily prescribed in personalized amounts based on each patient’s requirements. The main limitations of this dosage form are the risk of inaccurate dosing, poor stability of active ingredients in liquid formulations, and the need for preservatives that may be toxic to children. Extemporaneously prepared medications are dosages that are created in specialized compounding pharmacies. They are tailor made to meet the specific demands of patients, such as the dose amount needed, preferred mode of consumption (liquids, powders or tablets), and compatibility of excipients. Despite their many advantages in treating children they are subject to limitations like reduced accessibility, altered drug dissolution properties, and poor long-term stability (Falconer and Steadman, 2017, Zuccari et al., 2022).
Recently, a number of studies have attempted to address the limitations of traditional pediatric medicine by improving existing drug product forms and developing new technologies for dosage production. Some key innovations include the creation of scoring-friendly tablets, solid formulations that can be transformed into liquid suspensions if necessary, and devices such as modified feeding bottles and pacifiers to make administration of liquid medications easier. Development of novel technologies such as orodispersible films, mini-tablets and chewable tablets hold great potential for future drug delivery solutions (Lopez et al., 2015). Orodispersible films are drug-containing polymer films that can be directly administered into the patient’s mouth. There they disintegrate rapidly on the tongue and release the dose without requiring any additional fluid intake, and are thus ideal for patients with swallowing difficulties. They can be manufactured using various techniques such as casting the drug formulation into a film (solvent casting), or printing it onto an existing film substrate (inkjet printing) etc. Orodispersible films also support dosage personalization be enabling caregivers to dispense a specific length of film that corresponds to the desired dose strength. This technology however has limited capability in delivering dosages with high drug strengths and delayed release behavior (Jamróz et al., 2017, Öblom et al., 2019). Drug strength here is defined as the amount of active ingredient present in the drug product, this is alternatively termed as drug loading or potency in literature.
Pharmaceutical mini-tablets are one of the more promising novel dosage forms. They are small form drug products, with a diameter of < 4mm, that are suitable for children over the age of 1. Mini-tablets can be dispensed as single or multiple units, thereby allowing for dose flexibility. Furthermore, they can also incorporate features of regular-sized tablets such as delayed or sustained drug release, long term product stability, and taste masking etc. (Zuccari et al., 2022). While some studies have found mini-tablets to be the most acceptable dosage form for children (Van Riet-Nales et al., 2013), a consensus on this has not been reached (Miyazaki et al., 2022). Manufacturing of mini-tablets can however be challenging due to the poor powder flow properties of many active pharmaceutical ingredients (APIs). This limits the preparation of homogeneous formulation blends and transfer of the blends in uniform quantities into tableting dies. As a result, production of low dose medication and small-size dosages is particularly challenging.
Recently, several novel manufacturing techniques have been developed to address these limitations and facilitate the production of mini-tablets. These include conventional operations like direct compression and granulation, and innovative methods like 3D printing. Direct compression closely resembles the traditional drug product manufacturing approach: the API is blended with an excipient mixture and the resulting formulation is compressed into mini-tablets using special dies. Commercialization of this technique is relatively simple as many production sites already possess the equipment and expertise to use it. As previously discussed, the main drawback of this method is a need for excellent powder flow properties and resistance to segregation in the API-excipient powder blend, in order to produce dosages with consistent drug strength (Lura et al., 2019, Stoltenberg and Breitkreutz, 2011). Tabletability of some poorly flowing APIs can be improved by using techniques like spherical crystallization to increase particle size and improve powder flow properties (Chen et al., 2020). Additionally, various 3D printing techniques have been developed for manufacturing mini tablets, such as hot melt extrusion, fused deposition modeling, and pressure assisted microsyringe etc. In hot melt extrusion, the API and excipient powders are extruded into a thin filament, which is then cut into desired sizes to yield dosages. In fused deposition modeling, instead of cutting, the extruded filament is melted and printed into desired shapes. This gives greater control on the content uniformity and shape of the dosage unit. In pressure assisted microsyringe, the API-excipient formulation is mixed with a liquid binder to form a paste. This paste is then deposited layer-wise using a syringe to build the dosage. Although extrusion-based methods can produce dosages with high drug strengths, they are limited by high operating temperatures (which may cause degradation of the API) and possible lack of control on the final polymorphic form of API crystals generated (El Aita et al., 2020, Krause et al., 2021; S. Alshetaili et al., 2016).
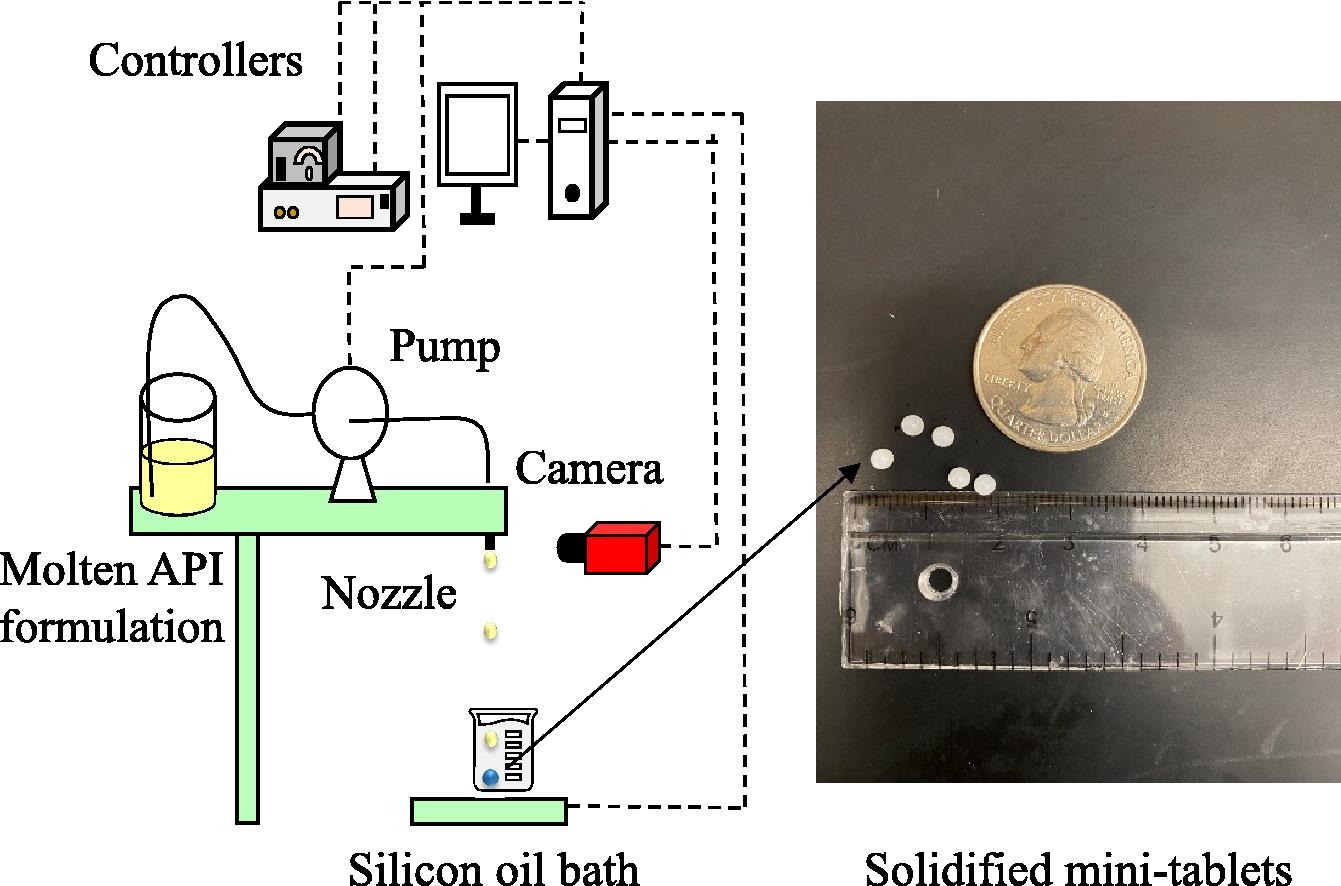
This study introduces a novel technique to manufacture pharmaceutical mini-tablets by using drop-on-demand (DoD) printing. DoD is an inkjet-type 3D printing technique that processes liquid formulations to produce drug products by printing several API containing droplets onto substrates like placebo tablets, capsules, and edible films. It is a versatile technique that can process a variety of formulations including solutions, melts and suspensions (Hirshfield et al., 2014; Içten, Giridhar, et al., 2015; Radcliffe et al., 2019). This study uses a melt-based formulation, in which the API is dissolved or suspended in a molten carrier fluid excipient. A DoD printer system is then employed to generate precise droplets of this formulation, which are subsequently captured and solidified in an inert solvent bath to form mini-tablets. Manufacturing mini-tablets using this approach has several advantages: 1) By processing the API as a liquid formulation, many of the powder handling challenges are avoided. 2) The DoD system generates droplets with uniform volumes, which in-turn translates into dosages with high content uniformity as errors associated with filament cutting and extrusion are eliminated. 3) The DoD platform facilitates the incorporation of emerging innovations in pharmaceutical manufacturing like continuous processing, real time quality assurance, automation, and process analytical technology etc. (Hirshfield et al., 2015, Sundarkumar et al., 2022b, Sundarkumar et al., 2022a). This helps align the resulting mini-tablet manufacturing process with future production standards that the industry is striving to achieve. In this study, first a framework for designing the solidification solvent bath is developed. Next, the manufacturing process is demonstrated for different API-excipient systems. Following this, the size, weight, drug strength, and dissolution behavior of the produced mini-tablets are analyzed to determine dosage uniformity. Lastly, promising areas of application, limitations, and future improvement directions for this technology are discussed.
Read more here
Varun Sundarkumar, Wanning Wang, Zoltan Nagy, Gintaras Reklaitis, Manufacturing pharmaceutical mini-tablets for pediatric patients using drop-on-demand printing, International Journal of Pharmaceutics, 2023, 123355, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123355.
Read more on Overview of Pharmaceutical 3D printing here:


