Formulation and process strategies to minimize coat damage for compaction of coated pellets in a rotary tablet press: A mechanistic view
Compaction of multiple-unit pellet system (MUPS) tablets has been extensively studied in the past few decades but with marginal success. This study aims to investigate the formulation and process strategies for minimizing pellet coat damage caused by compaction and elucidate the mechanism of damage sustained during the preparation of MUPS tablets in a rotary tablet press. Blends containing ethylcellulose-coated pellets and cushioning agent (spray dried aggregates of micronized lactose and mannitol), were compacted into MUPS tablets in a rotary tablet press.
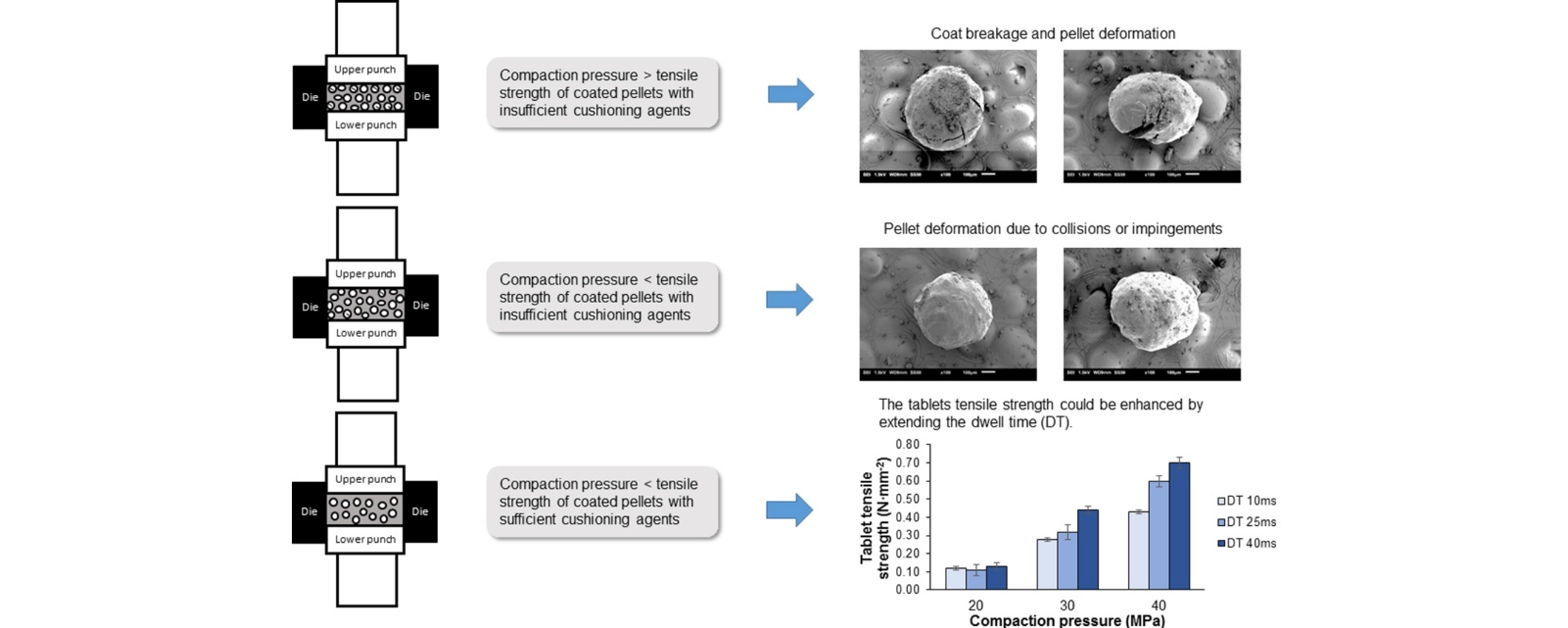
The effects of compaction pressure and dwell time on the physicomechanical properties of resultant MUPS tablets and extent of pellet coat damage were systematically examined. The coated pellets from various locations at the axial and radial peripheral surfaces and core of the MUPS tablets were excavated and assessed for their coat damage individually. Interestingly, for a MUPS tablet formulation which consolidates by plastic deformation, the tablet mechanical strength could be enhanced without exacerbating pellet coat damage by extending the dwell time in the compaction cycle during rotary tableting.
See pharm-a-spheres™ solution for MUPS here:
However, the increase in compaction pressure led to faster drug release rate. The location of the coated pellets in the MUPS tablet also contributed to the extent of their coat damage, possibly due to uneven force distribution within the compact. To ensure viability of pellet coat integrity, the formation of a continuous percolating network of cushioning agent is critical and the applied compaction pressure should be less than the pellet crushing strength. Find out more here…
Article information: Min Xu, Paul Wan Sia Heng, Celine Valeria Liew. International Journal of Pharmaceutics, 2016. https://doi.org/10.1016/j.ijpharm.2015.12.068.
Interested in a sample or more information of sugar spheres?
Materials
Lactose (Granulac 200, Meggle Pharma, Germany), mannitol (Man; Mannitol 35, Roquette, France), sugar cores (355–425mm; Pharm-a-spheres, Hanns G. Werner, Germany), hydroxypropyl methylcellulose (VLV, Dow Chemical, USA), polyvinylpyrrolidone (Plasdone C-15, Ashland, USA), ethylcellulose (EC) dispersion (Surelease, Colorcon, USA), magnesium stearate, cross-linked polyvinylpyrrolidone (Polyplasdone XL, Ashland, USA) and isopropyl alcohol (IPA, Aik Moh Paints and Chemicals, Singapore) were used as supplied. Deionized water (Milli-Q, Millipore Corporation, USA) was used when water was needed and metformin hydrochloride (USP grade, Granules India, India) was the model drug.

