A Multi-Particulate Formulation of Pantoprazole Based on Enteric Coated Minitablets

JRS Pharma presented three posters at the 2024 PBP World Meeting in Vienna. This is the first poster:
Introduction
Multiparticulate systems provide solutions to some of the main challenges found with gastro-resistant formulations, namely dose dumping, unpredictable gastric retention time, and lack of swallowability. A system in which the API is distributed over a multitude of individually coated units, provides a good protection against drug1 dumping by premature coating breakage.
Due to their gastric disintegration into their much smaller, enteric-coated subunits, multiparticulate systems enablemore reproducible gastric retention times.
Finally, the coated units, when emptied out of a capsule orsachet, are easier to swallow than monolithic entericcoated tablets. This becomes an important factor in geriatric and paediatric care. The small size allows for an easier swallowing and adjusting of the dosage for the 2,3 young and elderly patients.
While most multiparticular systems are based on pellets as the API-carrier, minitablets offer similar advantages, albeit at vastly reduced production complexity. In addition, their well defined specific surface area allows for more efficient and more consistent coating processes.
Aim of the Study
Aim of the study was to formulate a 20 mg multiparticulate, delayed release dosage form of pantoprazole, by mini tabletting, coating and encapsulation. Dissolution profiles were measured both in an acidic environment (pH 1 and pH 4.5) and subsequently in phosphate buffer (pH 6.8) to prove the functionality of the enteric coating and hence the formulation concept.
Material and Methods
A high functionality excipient composite (PROSOLV® EASYtab SP) consisting of microcrystalline cellulose, silicon dioxide, sodium stearyl fumarate and sodium starch glycolate, sodium stearyl fumarate (PRUV®) as well as the tablet subcoating PS-1P-041 were provided by JRS PHARMA GmbH & Co. KG (Rosenberg, Germany).
Furthermore, anenteric coating comprised of amethacrylic acid – ethyl acrylate copolymer, talcum, polyethylen-glycole, sodium bicarbonate and hydrogenated castor oil was provided by JRS PHARMA GmbH & Co. KG (Rosenberg, Germany). Pantoprazole sodium sesquihydrate was purchased from Everest Organics Limited (Sangareddy, India) and trisodium phosphate was purchased from Acros Organics B.V.B.A. (Waltham, U.S.A.). HPMC capsules (EMBO CAPS®-VG-Pro) with the size 0 were obtained from Suheung Europe GmbH (Eschborn, Germany).
Formulation
The quantitative composition of the formulation used for the production of minitablets is shown in Table 1. The ingredients except PRUV® were blended for 20 min at 100 rpm using a Micromix, without chopper, from the company Syntegon (Waiblingen, Germany). After the addition of the sieved PRUV®, the formulation was blended for 3 min at 24 rpm using a freefall blender Brunimat Type Porta (Brunitec Suisse, Ermatingen, Switzerland).

Tableting, Coating Process and Capsule Filling
Minitablets were pressed on a rotary tablet press of the type Pressima from the manufacturer IMA Kilian (Köln, Germany). Each punch set had ten tips with a diameter of 3 mm and a curvature radius of 2.8 mm. Compression force was adjusted to 1 kN for the precompression force and to 15 kN for the main compression force.
8 w/w % subcoating and 15 w/w % enteric coating were applied using a fluid bed Solidlab2 from Syntegon Technology GmbH (Waiblingen, Germany).
15 Minitablets were filled into each HPMC capsule using a capsule filling machine of the type GKF HiProTect 720 from the manufacturer Syntegon (Waiblingen, Germany). Filling speed was set to 15 cycles per minute.
Functional Tablet Characteristics
The crushing strength of the tablet cores was measured
with the model TBH 425 TD from Erweka GmbH (Langen, Germany).
The dissolution times for the coated minitablets filled in
capsules were analyzed using a PTWS 100 (Pharma Test Apparatebau AG, Hainburg, Germany).
Dissolution testing in acidic environments (pH 1 and pH 4.5) was followed by a medium change to phosphate buffer (pH 6.8) according to Ph.Eur. 5.17.1.
Results and Discussion
Tablet Cores

The PROSOLV® EASYtab SP based formulation resulted in robust minitablets that were suitable for a subsequent coating step when a compression force of 15 kN was applied. During tableting, ejection force was in an acceptable range of around 350 N as shown in Table 2.
Tablet Cores
The PROSOLV® EASYtab SP based formulation resulted in robust minitablets that were suitable for a subsequent coating step when a compression force of 15 kN was applied.
During tableting, ejection force was in an acceptable range of around 350 N as shown in Table 2.
Dissolution Profiles
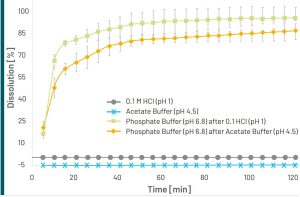
The dissolution profiles of the pantoprazole minitablets showed no API release for acidic environments at pH 1 and pH 4.5 indicating the robustness of the applied enteric coating in both the fasted and fed state stomach.
After a medium change to pH 6.8, there was a strong increase in API liberation within the first 10 – 20 min both for minitablets having been previously transferred to acidic media with pH 1 and pH 4.5 (Figure 2).
Conclusion
Formulation of pantoprazole minitablets was feasible with PROSOLV® EASYtab SP. Tablets were suitable for a subsequent enteric coating step in terms of mechanical robustness.
In order to reach an API content similar to conventional pantoprazole tablets, minitablets were filled in capsules.
Dissolution profiles measured in acidic media (pH 1 and pH 4.5) showed no API release whereas after a medium switch to phosphate buffer a high API release rate was measured within 10 – 20 min.
Download the full poster on “Multi-Particulate Formulation of Pantoprazole” here
(click on the image to see them enlarged)
Source: JRS Pharma, PBP World Meeting, poster “Multi-Particulate Formulation of Pantoprazole“, Sabine Wetzel¹; Alexander Altmann²; Gernot Warnke³; Isabelle Hemming⁴; Philipp Stirm⁵; Thomas Brinz⁶, JRS Pharma GmbH & CO. KG, Holzmühle 1, 73494 Rosenberg, Germany, Sabine.Wetzel[at]jrspharma.de, Syntegon Technology GmbH, Stuttgarter Straße 130, 71332 Waiblingen, Germany, Thomas.Brinz[at]syntegon.com
Do you need more information or a sample of JRS Pharma excipients?


